Search results with tag "Thiosulfate"
DBL SODIUM THIOSULFATE INJECTION Name of …
www.medsafe.govt.nzVersion 4.0 Page - 1 - DBL™ SODIUM THIOSULFATE INJECTION . Name of medicine Sodium thiosulfate . Presentation DBL™ Sodium Thiosulfate Injection is a clear, colourless, sterile solution. Each 10 mL vial contains 2.5 g of sodium thiosulfate …
MATERIAL SAFETY DATA SHEET Sodium thiosulfate
www.ch.ntu.edu.twMATERIAL SAFETY DATA SHEET Sodium thiosulfate Section 1 Chemical Product and Company Identification MSDS Name: Sodium thiosulfate
6 Determination of Hypochlorite in Bleach W10
www.kbcc.cuny.eduflask. Begin the titration promptly after adding this solution to the mixture. 8. Begin titrating the bleach solution with sodium thiosulfate solution. Allow about 2–3 mL of the thiosulfate solution to run into the reaction flask and close the stopcock. Don’t write the volume down at this point. You’ll be adding more thiosulfate in a few ...
Calcium Thiosulfate - Crop Vitality
www.cropvitality.comGENERAL INFORMATION CaTs® is a neutral to basic, chloride-free, clear solution, containing 6% calcium and 10% thiosulfate sulfur. Each gallon of CaTs contains 0.63 pound of calcium (Ca) and 1.0 pound of thiosulfate sulfur (S).
Safety Data Sheet SODIUM THIOSULFATE SOLUTION
ictulsa.comSafety Data Sheet SODIUM THIOSULFATE SOLUTION 1 Section 1 - Product and Company Identification Product Name: Sodium Thiosulfate Solution Chemical Formula: Na2S2O3 CAS Number: 00772-98-7 General Use: Waste water dechlorination agent and lab reagent Supplier: 701 N Post Oak Rd., Ste. 540 Houston, TX 77024 Section 2 - Hazards Identification ...
Reaction Kinetics: the Iodine Clock Reaction
www.fountainheadpress.com(sodium thiosulfate), and 5% starch indicator. Equipment: ... As long as there is any thiosulfate in solution, there is no triiodide to react with the starch indicator. Once all the thiosulfate has been consumed, Reaction (2) ceases. ... from Table 2 of the Data Sheet, calculate the reaction rates for experiments 9 and 10. .: ...
Analysis of Cyanide (Total, Weak Acid Dissociable, and ...
www2.gov.bc.caSodium thiosulfate can also be used instead of sodium arsenite. Add small portions (0.02 g/L), with re-testing after each addition. Do not add excess sodium thiosulfate. To determine chlorine < 2 mg/L use a DPD colourimetric method (APHA 4500-Cl.G) and add a stoichiometric amount of sodium thiosulfate solution (APHA 4500- Cl.B.2d). Note: If the ...
Student safety sheets 35 Sodium sulfites, thiosulfate ...
science.cleapss.org.ukStudent Safety Sheets are teaching materials. For safety management, use Hazcards and other resources on the CLEAPSS website. ©CLEAPSS 2019 Student safety sheets 35 Sodium sulfites, thiosulfate & persulfate including metabisulfite & …
GOLD SODIUM THIOSULFATE - SmartPractice
www.smartpractice.comGold sodium thiosulfate is a fairly common sensitizer with elicitation of symptoms linked to gold in jewelry, occupational exposure to gold, dental restorations, gold-plated intracoronary stents and previous rheumatoid arthritis treatment.
ANALYSIS OF BLEACH BY THIOSULFATE TITRATION
seniorchem.comThe determination of free chlorine in bleach is possible by a redox titration. The most common and successful method for use in high schools involves taking the sample of bleach converting the ... product is titrated with thiosulfate. As the iodine is used, the brown colour due to the tri-iodide ion fades to yellow, and then disappears. This is ...
An iodine / thiosulfate titration
mccscience.yolasite.comAn iodine / thiosulfate titration Theory Aqueous iodine solutions normally contain potassium iodide (KI), which acts to keep the iodine in solution. This is due to the fact that an equilibrium is set up as follows: I2 + I - I 3-I3 - is much more soluble than I 2 and it is as I 3 - …
2.37H TITRATIONS WITH SODIUM THIOSULFATE - Cengage
www.cengage.comThe titration of iodine by thiosulfate tends to yield slightly low results owing to the adsorption of small but measurable quantities of iodine on solid CuI.
Student safety sheets 35 Sodium sulfites, thiosulfate ...
science.cleapss.org.ukSodium sulfites, thiosulfate & persulfate including metabisulfite & potassium salts Substance Hazard Comment Sodium & potassium sulfite [sulfate(IV)]; sodium & potassium metabisulfite [disulfate(IV)] Solid and concentrated solution (If 0.15 M or more) HARMFUL CORROS. D. ANGER: Harmful if swallowed, cause serious eye damage. ...
Sodium Thiosulfate, Pentahydrate(10102-17-7)
www.labchem.comSodium Thiosulfate, Pentahydrate Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 03/07/2005 Revision date: 04/25/2018 Supersedes: 07/09/2013 Version: 1.1 04/25/2018 EN (English US) Page 1 SECTION 1: Identification 1.1. Identification
Determination of Ethanol Concentration in Aqueous Solutions
www.canterbury.ac.nziodine complex. Figure 4 As more thiosulfate is added and we near the titration endpoint, the blue-black colour from the starch-iodine complex fades. Figure 5 The endpoint of the titration is reached when just enough thiosulfate is added to react with all the iodine present and the solution becomes colourless. 2 Method Sample Preparation 1.
Chemistry 120: Experiment 5
www1.udel.eduThe triiodide formed in this reaction is titrated with the standard thiosulfate solution that was prepared earlier. 2 S2O3 2– + I 3 – = S 4O6 2– + 3 I– (4) thiosulfate ↑ ↑ tetrathionate The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. 1.
TITRATION OF ACTIVE CHLORINE WITH SODIUM …
www.antenna.ch: Volume of the sodium thiosulfate solution used for the titration in ml c Na 2 S 2 0 2: Concentration of thiosulfate: = 0.1 mol/l V échant. : Volume of the sample of sodium hypochlorite = 5 ml M Cl : Molar atomic mass of chlorine = 35.4527 g/mol m …
REDOX TITRATION - Weebly
chembloginc.weebly.comProperly fill a burette with the thiosulfate solution. 4. Titrate with the thiosulfate until the solution has lost its reddish-brown color and has become orange. 5. Add 2 mL of starch indicator and complete the titration. 6. Note the initial and final burette readings to at …
MATERIAL SAFETY DATA SHEET - Liquid Bromine
liquidbromine.com1.10-50% potassium carbonate solution. 2.10-30% sodium carbonate solution. 3.5-10% sodium bicarbonate solution. 4.Sodium thiosulfate solution (prepared by dissolving 4kg of technical grade sodium thio sulfate in 9 litre of water and adding 100gr of soda ash) please note that there is a high heat of reaction release in this
Core practical 13a: Follow the rate of the iodine ...
qualifications.pearson.comSodium thiosulfate releases sulfur dioxide when it reacts. Ensure that the room is well-ventilated. Propanone is an irritant and is highly flammable. Sulfuric acid solution is an irritant. Do not run this practical for more than 12 minutes in total. Practical techniques 1, 4, 11, 12 CPAC 1a, 2a, 2b, 3a, 4a, 4b
PROCESSING THE RADIOGRAPH - Columbia University
www.columbia.eduGold and amalgam have high z values. 4 4 The X-ray film is a delicate product, sensitive to many things, e.g. light photons, X-rays and ... Sodium Thiosulfate (hypo) Removes unexposed crystals . 5 5 Ammonium Thiosufate [Removes all crystals] b.
Determination of Vitamin C Concentration by Titration
www.canterbury.ac.nz3. The concentration of the prepared iodine solution can be more accurately determined by titration with a standard solution of ascorbic acid or a standard solution of potassium thiosulfate using a starch indicator. This should be done if possible as iodine solutions can be unstable. 4. The average titre volume should ideally be in
METHOD 350.1 DETERMINATION OF AMMONIA NITROGEN …
www.epa.govthiosulfate or other reagents before distillation. 4.3 Method interferences may be caused by contaminants in the reagent water, reagents, glassware, and other sample processing apparatus that bias analyte response. 5.0 SAFETY. 5.1 The toxicity or carcinogenicity of each reagent used in this method have not been fully established.
4500-Cl CHLORINE (RESIDUAL)* 4500-Cl A. Introduction
edgeanalytical.comThe amperometric titration method (D) is a standard of com-parison for the determination of free or combined chlorine. It is ... iodine, permanganate, hydrogen peroxide, and ozone. How- ... thiosulfate may be used as the standard reducing reagent at pH 4.
The Determination of the Iodine Number of
www.drcarman.info3 The I 2 is then titrated with a standard solution of sodium thiosulfate as performed in the redox titration in CHEM 121: I 2 + 2 Na 2 S 2 O 3 2 NaI + Na
UNITS OF CONCENTRATION - Vancouver Island University
web.viu.ca2-, each mole of thiosulfate is involved in the transfer of 1 equivalent of e- in reacting to form S4O6 2-. Thus K = 1. Conversion Chart for Concentrations of O2 TO Æ FROM mg O2 /L moles O2/L equiv O2 /L mg O2 /L - 1 mol/32,000 mg 1 equiv/8000 mg moles O2/L 32,000 mg/mol - 4 equiv/mol equiv O2/L 8000 mg/equiv 1 mol/4 equiv -
List of Common Ions - Chemistry
www.chalmerschemistry.weebly.comList of Common Ions Polyatomic Cations NH4 + ammonium H3O + hydronium Polyatomic Anions OH-hydroxide CN-cyanide O2 2-peroxide CO3 2-carbonate C2O4 2-oxalate NO2-nitrite NO3-nitrate PO3 3-phosphite PO4 3-phosphate SO3 2-sulfite SO4 2-sulfate S2O3 2-thiosulfate ClO-hypochlorite ClO2-chlorite ClO3-chlorate ClO4-perchlorate CH3COO or C2H3O2-acetate …
Common Ions and Their Charges - ScienceGeek.net
www.sciencegeek.netthiosulfate ammonium hydronium ... calcium barium Fr+ francium Ra2+ radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3+ aluminum Si silicon phosphide P3-sulfiide S2-chloride Cl-argon Ar helium He Zn2+ zinc In3+ indium Ge4+ germanium As3-
PROHIBITED AND RESTRICTED CHEMICAL LIST - Washington, …
esa.dc.govSodium Phosphate, Tribasic Sodium Potassium Alloy Sodium Sulfide Sodium Thiocyanate Sodium Thiosulfate Stannic Chloride Strontium Nitrate Sulfur Chloride Sulfur Pentafluoride Sulfuric Acid «10%) Sulfuric Acid (>10%) T -Butanol Terpineol Thiophosphoryl Chloride Tin Toluene Toluene Diisocyanate Toluidine Blue Trichloroethane, 1,1,1-
Redox Titration - University of Massachusetts Boston
alpha.chem.umb.eduRedox Titration • Redox titration is based on the redox reaction (oxidation-reduction) between analyte and titrant. Ce. 4+ ... or Soduim Thiosulfate (Na. 2. S. 2. O. 4) for standardization • Storage, no light, no oxygen . Properties of Umass Boston . Properties of Umass Boston .
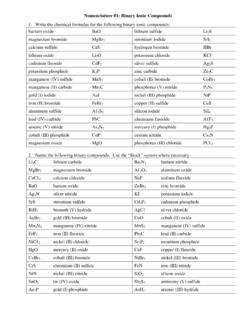
Nomenclature #1: Binary Ionic Compounds - Patterson Science
pattersonscience.weebly.comarsenic (V) thiosulfate As 2(S 2O 3) 5 gold (III) fluoride AuF 3 calcium permanganate Ca(MnO 4) 2 sodium peroxide Na 2O 2 aluminum thiocyanate Aℓ (SCN) 3 strontium cyanate Sr(OCN) 2 copper (II) hydrogen carbonate Cu(HCO 3) 2 lead (IV) hypoiodite Pb(IO) 4 silver dichromate Ag 2Cr 2O 7 iron (III) borate FeBO 3
Ionic Charges Chart (Cations and Anions) Cations
assets.gpb.orgsodium Na+ copper(I) Cu ... gold(III) Au3+ iron(III) Fe3+ manganese(III) Mn3+ 4+ tin(IV ... thiosulfate S 2 O 3 2-3- arsenate AsO 4 3-arsenite AsO 3 3- citrate C 6 H 5 O 7 3- ...
Production of Gold - West Virginia University
cbe.statler.wvu.edupure and are submerged with 99.9% pure rolled gold cathodes in an electrolytic solution with 100g/L each of gold and hydrochloric acid. A current density of 800 A/m2 is applied at 60°C, and the gold collected on the cathodes is rinsed several times with a hot sodium thiosulfate solution before the 99.9% pure cathodes are melted and recast as final
CH COO TABLE OF POLYATOMIC IONS arsenate ... - …
www.sciencegeek.netthiosulfate ammonium hydronium C2O4 2– ClO4 – IO4 – MnO4 – O2 2– PO4 3– P2O7 4– SO4 2– SO3 2– SCN– S2O3 2– NH4 + H3O + POSITIVE POLYATOMIC IONS TABLE OF POLYATOMIC IONS H2PO4 – HCO3 – HC2O4 – HSO4 – HS– HSO3 – OH– ClO– IO3 – HPO4 2– NO3 – NO2 – SiO4 4– hydrogen carbonate hydrogen oxalate hydrogen ...
BACKGROUND AND PRINCIPLE - Indian Institute of …
web.iitd.ac.inPage 8 Standard sodium thiosulfate titrant: Dissolve 6.205 g Na 2S2O3 . 5H 2O in distiller water and add 1.5 mL 6N NaOH or 0.4 g solid NaOH and dilute to 1000 mL. Standardize with bi-iodate solution. PROCEDURE
Similar queries
Thiosulfate, Sodium thiosulfate, Company Identification, Titration, Calcium Thiosulfate, Safety Data Sheet SODIUM THIOSULFATE SOLUTION, Sodium thiosulfate solution, Reaction Kinetics: the Iodine Clock Reaction, Solution, Data Sheet, Sodium, Student safety sheets 35 Sodium sulfites, thiosulfate, Student Safety Sheets, Safety, Gold sodium thiosulfate, Bleach, THIOSULFATE TITRATION, Iodine, An iodine / thiosulfate titration, Sodium sulfites, thiosulfate, SAFETY DATA SHEET, Sodium thio sulfate, Columbia University, Gold, Ions, Calcium, Redox titration, Compounds