Search results with tag "Titrations"
Back Titrations - chemistryattweed
www.chemistryattweed.comBack titrations are used when: - one of the reactants is volatile, for example ammonia. - an acid or a base is an insoluble salt, for example calcium carbonate - a particular reaction is too slow - direct titration would involve a weak acid - weak base titration (the end-point of this type of direct titration is very difficult to observe)
Required Practical 1 - PMT
pmt.physicsandmathstutor.comsubstances that have acid-base properties and could affect the reaction. If they don’t have acid-base properties we can titrate with confidence. Testing batches: In quality control it will be necessary to do titrations or testing on several samples as the concentration and amount of the chemical being tested may vary between samples.
Experiment 7 - Acid-Base Titrations
www.lahc.eduIn an acid-base titration, the neutralization reaction between the acid and base can be ... Four lab periods assigned for this experiment. In part I you will prepare an acid (HCl) solution and a base ... Chemistry 101: Experiment 7 Page 2 the flask. Stopper the flask and shake to mix. The solution should be approximately 0.2 N HCl.
84.315 Analytical Chemistry I Laboratory Manual
faculty.uml.edu5 Evaluation of an antacid by volumetric titration COMPEXATION)TITRATIONS) 6 a) Determination of Ca by displacement titration ... Precipitation titration: Determination of percentage Cl-by Mohr's method. b) ... instrumental technique used, instrument used, and the result of the analysis of the unknown. This is not an
Common Apparatus and Procedures - Chem Lab
chemlab.truman.eduamount of acid equals the amount of base. The experimenter is aware of this condition by the change in pH that occurs. The change in pH is indicated by a color change of an indicator or by a pH meter. In other types of reactions, the completion of the reaction is usually also indicated by a color change. Titrations are often accomplished using ...
Redox titrations with potassium permanganate
teaching.ch.ntu.edu.twThe redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The balanced chemical reaction equation
Ch. 11: EDTA Titrations
chem320.cs.uwindsor.caanalogous to plotting pH versus volume of titrant in an acid-base titration. There are three natural regions of the titration curve. Three regions in an EDTA titration illustrated for reaction of 50.0 mL of 0.0500 M Mn+ with 0.0500 M EDTA, assuming K fʹ = 1.15 × 1016. The concentration of free Mn+ decreases as the titration proceeds.
EDTA Titrations 1: Standardization of EDTA and Analysis of ...
www.uclmail.netAn EDTA solution of unknown molarity was standardized by titration of 10.00 mL (0.01000 L) samples of the standard zinc ion solution of Example 1 by the method of this experiment. The mean corrected titration volume of the EDTA solution was 16.25 mL (0.01625 L).
REDOX TITRATION - Weebly
chembloginc.weebly.comredox titrations: I2 + I-→ I 3-. The actual reaction that occurs in the redox titration is then between the tri-iodide ion and the thiosulphate ion. In this experiment, the thiosulphate is titrated against a known volume of a standard iodate in the presence of excess iodide. The endpoint is signaled by the disappearance of a blue
ACID BASE TITRATION OBJECTIVES INTRODUCTION
www.augusta.eduACID BASE TITRATION OBJECTIVES 1. To demonstrate the basic laboratory technique of titration 2. To learn to calculate molarity based on titrations INTRODUCTION Molarity (M) or molar concentration is a common unit for expressing the concentration of solutions.
Bloom's Taxonomy of Educational Objectives
teaching.charlotte.edutitrations on a series of unknown acids and find a variety of problems with the resulting curves, e.g., only 3.0 ml of base is required for one acid while 75.0 ml is required in another. What can you do to get valid data for all the unknown acids? Organization Creates new tasks or objectives incorporating learned ones Recall your plating and
EXPERIMENT 1: HARDNESS OF WATER BY EDTA TITRATION …
www.calstatela.eduChemistry 201 Laboratory Fall 2008 Page 2 of 3 In3-is orange).Some metal ions (commonly Fe3+ and Cu2+) interfere with the endpoint by causing fading or indistinct endpoints. This interference can be eliminated by adding appropriate inhibitors, one of which is the hydroxylamine hydrochloride added to the indicator solution in this procedure.
Investigating Vitamin C
www.york.ac.ukvitamin C. It should be possible to carry out an acid /base type titration because vitamin C is an acid. Sources of Information • Denby D., There’s more to Vitamin C than Brussel Sprouts C hemistry Review , May 1996 • Selinger B., (1998), Chemistry in the Marketplace , Harcourt, Brace, Jovanovich, London
Experiment 5 - Synthesis of Aspirin
lahc.eduCompare the melting point of you aspirin product to the theoretical melting point (138-140 C). Is the crude product above of below this mark? Explain why this is the case. Determine the moles of aspirin from the titration and calculate the percent purity of the crude aspirin product from the titration analysis.
Determination of Aspirin using Back Titration
www1.lasalle.edu3. Ethanol was used in the solutions to help dissolve the acetylsalicylic acid. Ethanol is slightly acidic, and will react with NaOH. Describe a blank correction experiment; i.e., what experiment you might do to determine how much NaOH reacts with the ethanol in your solution. Once you have determined this,
Determination of Aspirin using Back Titration
mccord.cm.utexas.eduDetermination of Aspirin using Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. You will ... Accurately record the weight of a group of three aspirin tablets so that you can determine an average tablet weight. Use a mortar and pestle to crush enough tablets to produce ~ 1 g tablet ...
iBreeze Series CPAP - Resvent
www.resvent.comCompact design with friendly UI for all your convenience. Continuous water level monitoring can maximize safety with plug and seal intelligent humidifier.. Unique IPR (Intelligent pressure release) algorithm offer more comfortableness during ventilation therapy.. CPAP-V allows patient for pressure titration within predetermined time..
6 Determination of Hypochlorite in Bleach W10
www.kbcc.cuny.eduflask. Begin the titration promptly after adding this solution to the mixture. 8. Begin titrating the bleach solution with sodium thiosulfate solution. Allow about 2–3 mL of the thiosulfate solution to run into the reaction flask and close the stopcock. Don’t write the volume down at this point. You’ll be adding more thiosulfate in a few ...
Practical Manual - Shri Ramdeobaba College of Engineering ...
rknec.eduIn this experiment a titration technique is used to measure the combined Ca2+ and Mg2+ concentrations in a water sample. The titrant or the intermediate solution is the disodium salt of ethylene diamine tetraacetic acid (abbreviated Na2H2Y or EDTA). Its structure is as below: NaOOC-H2C CH2-COOH \ / N-CH2-CH2-N / \
Fe Analysis by REDOX Titration - Community College of ...
www.ccri.eduFe Analysis by REDOX Titration Prestudy 1. Write the balanced net-ionic equation for the reaction of ferrous ion with permanganate in an acidic solution. Fe+2 + MnO 4-1-----> Fe+3 + Mn+2 2. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. What is the ...
CHEMICAL PRECIPITATION: WATER SOFTENING
www.egr.msu.edu9 Appendix -1 10 RAW DATA 10 Appendix-2 11 Sample Calculation to Determine the Lime Dosage 11 Appendix-3 13 ... Before Titration 16 Mid-Titration 16 End of Titration 16 Table 1: : Run1 for varying lime dosages 10 Table2: Run2 for varying lime dosages 10 . 1 ABSTRACT Hard water can cause many problems including scaling and excessive soap ...
Precipitation Titration: Determination of Chloride by the ...
academic.brooklyn.cuny.eduPrecipitation Titration: Determination of Chloride by the Mohr Method by Dr. Deniz Korkmaz Introduction Titration is a process by which the concentration of an unknown substance in solution is determined by adding measured amounts of …
Karl Fischer Titration - Mettler Toledo
www.mt.comMETTLER TOLEDO Good Titration PracticeTM in KF Titration 4 1 Fundamentals of the Karl Fischer titration 1.1 An historic overview 1935 Publication: "Neues Verfahren zur massanalytischen Bestimmung des
1092 THE DISSOLUTION PROCEDURE: DEVELOPMENT AND …
latam-edu.usp.orgThis chapter addresses the development and validation of dissolution procedures, with a focus on solid oral dosage forms. ... For products containing more than a single active ingredient, develop and validate the method(s) for each active ingredient. (USP 1-Dec-2020) ... (such as dynamic solubility, potentiometric titration, or turbidity ...
Hidden Workers: Untapped Talent - hbs.edu
www.hbs.eduOct 04, 2021 · time it takes for a new employee to achieve expected levels of productivity, attrition rates, and rates of advancement. Developing a customized approach to hiring hidden workers Shifting the justification for hiring hidden workers from corporate social responsibility (CSR) to ROI. A company that relegates a group
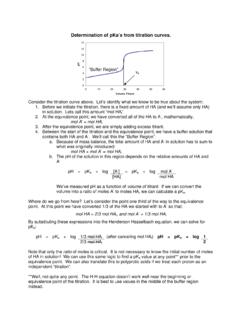
Determination of pKa from Titration Curve
blamp.sites.truman.eduConsider the titration curve above. Let’s identify what we know to be true about the system: 1. Before we initiate the titration, there is a fixed amount of HA (and we’ll assume only HA) in solution. Lets call this amount “mol HAi” 2. At the equivalence point, we have converted all of the HA to A-, mathematically, mol A-= mol HA i 3.
TITRATION OF ACTIVE CHLORINE WITH SODIUM …
www.antenna.ch: Volume of the sodium thiosulfate solution used for the titration in ml c Na 2 S 2 0 2: Concentration of thiosulfate: = 0.1 mol/l V échant. : Volume of the sample of sodium hypochlorite = 5 ml M Cl : Molar atomic mass of chlorine = 35.4527 g/mol m …
SOIL SURVEY LABORATORY METHODS MANUAL - USDA
www.nrcs.usda.govin analytical instrumentation and development of analytical methods. In 1995, a revised version (Version 3.0) of the SSIR No. 42 was initiated by Dr. M. Dewayne Mays, Head, Soil Survey Laboratory, to reflect these many changes. Many of the contributors to the earlier versions participated in the review ... 4 Titration 6A1a* 604 CO 2) 16) ...
Using Graphical Analysis in Titration Curves
www.ccri.eduUsing Graphical Analysis in Titration Curves A typical titration curve obtained by plotting the measured pH of a weak acid solution as a strong base is added using graphical analysis for Windows is illustrated as follows: The equivalence point is halfway up the vertical portion of the curve, about 27 mL for this titration.
Chapter 9
dpuadweb.depauw.edu9A.3 Titration Curves To find a titration’s end point, we need to monitor some property of the reaction that has a well-defined value at the equivalence point. For example, the equivalence point for a titration of HCl with NaOH occurs at a pH of 7.0. A simple method for finding the equivalence point is to monitor the
Titration curves, labelled E F G and H, for combinations ...
drwainwright.weebly.comIn an experiment to determine the acid dissociation constant (Ka) of a weak acid, 25.0 cm3 of an approximately 0.1 mol dm–3 solution of this acid were titrated with a 0.10 mol dm–3 solution of sodium hydroxide. The pH was measured at intervals and recorded. The table below shows the results. € € Volume of NaOH / cm 3 0.0 1.0 2.0 3.0 4.0 ...
Titration Guide on Errors - Environmental-Expert.Com
d3pcsg2wjq9izr.cloudfront.net• Precipitation (e.g. Argentometric titration) • Complexometric To ensure high-quality results, the following aspects of the method and apparatus should be considered. a) Titration beaker Cleanliness The first and most important rule for titration beakers is that they should be absolutely clean before using them for the analysis.
PREFORMULATION STUDIES: PHYSICOCHEMICAL …
www.ramauniversity.ac.infrom the area under DSC- curve for meltingendotherms. Similarly, heat of transition from one polymorph to another may be ... pKa is the dissociation constant of a drug ... potentiometric titration, titrimetric method 05/12/2015 NGSMIPS 30. SOLUBILIZATION
Guidelines for Manual Sampling & Analyses
www.indiaenvironmentportal.org.inyellow. Add 5 ml starch indicator solution and continue the titration until the blue colour disappears. Calculate the normality of the stock solution. • Sodium Thiosulphate Titrant (0.01 N) - Dilute 100 ml of the stock thiosulfate solution to 1 litre …
List of New Topics - ICSI - Home
icsi.eduEmployee Retention Practices in organisation/ Best practices in Attrition Management Recruitment Policies Job Satisfaction among employees Job Design Model of Motivation A Study of Human Resource Costing ... A Study on organisational Culture and its impact on employee behaviour A study on Employee Empowerment and its impact on Job Performance
Determination of Vitamin C Concentration by Titration
www.canterbury.ac.nz(Redox Titration Using Iodate Solution) Safety Lab coats, safety glasses and enclosed footwear must be worn at all times in the laboratory. Note: iodate is toxic by ingestion. Determination of Vitamin C Concentration by Titration. Equipment Needed burette and stand 100 mL volumetric flask 20 mL pipette 250 mL conical flasks ...
Determination of Vitamin C Concentration by Titration
www.canterbury.ac.nzThis method determines the vitamin C concentration in a solution by a redox titration using iodine. Vitamin C, more properly called ascorbic acid, is an essential antioxidant needed by the human body (see additional notes). As the iodine is added during the titration, the ascorbic acid is oxidised to dehydroascorbic acid, while
Redox Reaction - the basics
www.calstatela.eduRedox expressions Redox Titration Curve EXAMPLE: Derive the titration curve for 50.00 mL of 0.0500 M Fe2+ with 0.00, 15.00, 25.00, 26.00 mL 0.1000 M Ce4+ in a medium that is 1.0 M in HClO 4. Potential of saturated calomel electrode is 0.241 V.
Core practical 11: Find the amount of iron in an iron ...
qualifications.pearson.comusing redox titration Objectives To calculate the percentage of iron in an iron tablet To perform a redox titration involving Fe2+(aq) and MnO 4−(aq) Safety Use eye protection. 1.5 mol dm−3sulfuric acid is an irritant. All the maths you need Change the subject of an equation.
Lenti-X Concentrator Protocol-at-a-Glance
www.takarabio.comFeb 24, 2009 · NOTE: For fast determination, the Lenti-X qRT-PCR Titration Kit (Cat. No. 632165) directly quantitfies the viral genomes in your virus stock, which is much faster and often more useful than antibiotic selection. Since it exploits conserved regions contained in most lentiviral vectors, all particles, regardless of expression features
Titration Method Validation - USP
www.usp.orgMethod validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. Before the method validation, it is necessary to standardize the titrant, in order to achieve accurate results. Method validation for titration should include determination of the specificity, linearity, accuracy,
Factors Affecting Motivation among Employees in ...
ijesi.orgThis study would be a prelude and of great help to managers and Human Resources professionals to raise the productivity of the company by increasing the motivation of their employees. ... Motivation of an Employee : ... • There is a dip in the performance level of employees, coupled with higher attrition rates.
STRENGTH AND STABILITY TESTING FOR COMPOUNDED …
www.usp.orgJan 13, 2014 · Other methods used to test strength include titration, which uses the principles of chemistry, and microbial assays, which are sometimes used to test antibiotics. Titration is based upon a known ... The method validation confirms that the method meets certain criteria. The typical analytical characteristics used in method validation include ...
Karl Fischer Titration - Mettler Toledo
www.mt.comPolyols are raw materials for the production of a big variety of polyurethane-based products obtained by polymerization reaction with Di-Isocyanates. An example is foams, which can be used for sound insulation. It is important to know the exact water content of the polyol, since the water reacts with the isocyanate groups to form CO 2. The CO
A Guide to Titration - RACI
www.raci.org.au1.3 Titration curves In each case, 0.1 mol dm-3 (0.1 mol.L-1) base is being added to 50 mL of 0.1 mol dm-3 (0.1 mol.L-1) acid. 1. Strong base added to strong acid 2. Strong base added to weak acid 2 Rationale for procedures used in titration Titration is a procedure which a skilled practitioner can perform with a high degree of both
Experiment 7: Titration of an Antacid - Boston College
www.bc.edu1 Experiment 7: Titration of an Antacid Objective: In this experiment, you will standardize a solution of base using the analytical technique known as titration.Using this standardized solution, you will determine the acid neutralizing power of a commercially available antacid tablet.
Similar queries
Titrations, Base, Acid, Base titration, Chemistry, Analytical Chemistry I Laboratory Manual, Volumetric titration, Titration, Precipitation Titration, Analysis, Redox, Potassium permanganate, Redox titration, ACID BASE TITRATION OBJECTIVES, ACID BASE TITRATION OBJECTIVES 1, WATER BY EDTA TITRATION, C hemistry, Aspirin, Determination of Aspirin using Back Titration, Acetylsalicylic acid, Aspirin tablets, Tablets, IBreeze Series, Compact, Thiosulfate, Experiment, Fe Analysis by REDOX Titration, Karl Fischer Titration, Dissolution, Validation, Method, Employee, Attrition, Determination of pKa from Titration Curve, Curve, USDA, Instrumentation, Titration curves, Chapter 9, Acid dissociation constant, Solution, Precipitation, Study, On employee, Study on Employee, Of Vitamin C, Vitamin C, Lenti, Vectors, Titration Method Validation, Method validation, Titration method, Factors Affecting Motivation among Employees in, AND STABILITY TESTING FOR COMPOUNDED, Polyurethane, Foams, Titration Titration, Experiment 7: Titration of an Antacid, Boston College, Antacid