Search results with tag "Intermolecular"
Types of Intermolecular Forces - Everett Community College
www.everettcc.eduTypes of Intermolecular Forces W 317 Everett Community College Tutoring Center Student Support Services Program What is the strongest intermolecular force present for each of the following molecules?
Chapter 11 Intermolecular Forces, Liquids, and Solids
alpha.chem.umb.eduIntermolecular Forces • List the substances BaCl 2, H 2, CO, HF, and Ne in order of increasing boiling points. • The attractive forces are stronger for ionic substances than for molecular ones • The intermolecular forces of the remaining substances depend on molecular weight, polarity, and hydrogen bonding. The
ACADEMIC YEAR 2020-2021
tsbie.cgg.gov.inChapter 4 STATES OF MATTER: GASES AND LIQUIDS 4.1 Intermolecular forces 4.2 Thermal energy 4.3 Intermolecular forces Vs thermal interactions 4.4 The gaseous State. 4.5 The gas Laws 4.6 Ideal gas equation. 4.7 Grahams Law of diffusion – Dalton’s law of partial pressures 4.8 Kinetic molecular
STATES OF MATTER
ncert.nic.inthe natur e of inter molecular forces, molecular interactions and effect of thermal energy on the motion of particles because a balance between these determines the state of a substance. 5.1 INTERMOLECULAR FORCES Intermolecular forces are the forces of attraction and repulsion between interacting particles (atoms and molecules). This term
IMF Intermolecular Forces Worksheet
web.gccaz.eduIMF – Intermolecular Forces Worksheet Indicate the strongest IMF holding together thousands of molecules of the following. Then indicate what type of bonding is holding the atoms together in one molecule of the following. NOTE – if the molecule is an ionic compound, then there is no IMF, the ions are all held together by ionic bonds.
AP Chemistry Unit 2 Intermolecular Forces and Properties ...
tutorified-wp-bucket.s3-accelerate.amazonaws.comDetermine the type of intermolecular force present in SiO2. A.dipole dipole B.dispersion C.ionic D.covalent network Question 6 What is the basis of a metallic bond? A.the attraction of neutral metal atoms. B.the attraction between protons and neutrons. C.the attraction between positive metal ions and interlocking electrons. D.the attraction between positive metal ions and free …
Introduction to Intermolecular Forces
chemcenter.ucmerced.eduThe Effect of Intermolecular Forces Table 1: Physical Properties of non-polar Halogens Element F 2 Cl 2 Br 2 I 2 m.p. (°C) -220 -101 -7.3 114 b.p. (°C) -188 …
10 KINETIC THEORY OF GASES - National Institute of Open ...
www.nios.ac.inmolecules which are held together by intermolecular forces. At room temperature, these atoms/molecules have finite thermal energy. If thermal energy increases, molecules begin to move more freely. This state of matter is said to be the gaseous state. In this state, intermolecular forces are very weak and very small compared to their kinetic energy.
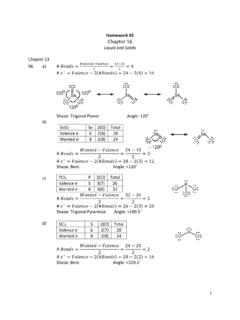
Homework #2 Chapter 16
people.chem.ucsb.eduintermolecular forces the smaller the vapor pressure. All solids also have a vapor pressure. This is why if you leave ice in the freezer for a long time it “disappears.” The vapor pressure of solids is less than the vapor pressure of liquids. As the temperature
Slide 1 Intermolecular Forces - ChemGod.com
chemgod.comSlide 13 Dispersion Forces are ALWAYS ATTRACTIVE All molecules like each other, at least a little bit. So all molecules stick together, at least a little bit.
Chapter 11 - Intermolecular Forces, Liquids and Solids
www.unf.edu&k /ltxlgv dqg ,qwhuprohfxodu )rufhv /hduqlqj jrdov dqg nh\ vnloov ,ghqwli\ wkh lqwhuprohfxodu dwwudfwlyh lqwhudfwlrqv glvshuvlrq glsroh glsroh k\gurjhq erqglqj
Laboratory: Intermolecular Forces (IMF)
natsci.parkland.eduExperiment 2: Drops on a penny 1. First practice using a dropper/pipet with a cup of water. Notice that if you put the dropper in the water first and then squeeze it, bubbles will come out. Instead, the
PowerPoint - Intermolecular Forces - Ionic, Dipole, London
ghsacceleratedchemistry.weebly.comIonic, Dipole - Dipole attractions •We have seen that molecules can have a separation of charge. •This happens in both ionic and polar bonds (the greater the EN, the greater the
Chemistry 1 Class Notes - Mr. Bigler
www.mrbigler.comTypes of chemical reactions. Predicting products. Intermolecular Forces (IMF) vs. physical state at room temperature 12, 14, 15 HS-PS1-3 IMF vs. bulk properties (melting point/boiling point, density, vapor pressure, etc.) 11, 12 HS-PS1-4 Energy of reaction, heat of formation 18 HS-PS1-5
Chemistry Notes for class 12 Chapter 11 Alcohols, Phenols ...
ncerthelp.com11 are oily liquids and higher members are waxy solids. 2. The hydroxyl groups in alcohols can form H-bonds with water, so alcohols are miscible with water. The solubility decreases with increase in molecular mass. 3. Boiling points of alkanes are higher than expected because of the presence of intermolecular hydrogen bonding in the polar ...
AP Chemistry Scoring Guidelines, 2016
secure-media.collegeboard.orgonly possible intermolecular forces are London dispersion forces. The London dispersion forces are stronger in I because it is larger in size with more electrons and/or a more polarizable electron cloud. The stronger London dispersion forces in I result in a higher melting point,which makes I 2 a solid at room temperature.
The Chemistry of Food Colorings - American Chemical …
www.acs.orgintermolecular forces. So when sugar dissolves in water, the attractive forces between the individual molecules are overcome, and these molecules are released into solution. Food-coloring molecules are usually ionic solids, that is, they contain positive and negative ions, which are held together by ionic bonds.
Factors that Affect the Rate of Dissolving and Solubility
arthurscience.weebly.com1. The forces holding the solute together must be broken (requires energy) Ionic compounds – the forces holding the ions together must be broken Covalent molecules – the forces holding molecules together must be broken 2. The intermolecular forces (between particles) holding the solvent together must be broken (requires energy) 3.
Physics Notes Class 11 CHAPTER MECHANICAL PROPERTIES …
ncerthelp.comViscous forces are intermolecular forces acting between the molecules of different layers of liquid moving with different velocities. where, (dv/dx) = rate of change of velocity with distance called velocity gradient, A = area of cross-section and = coefficient of viscosity. SI unit of η is Nsm-2 or pascal-second or decapoise.
Lecture 5: Coagulation and Flocculation
site.iugaza.edu.psThe magnitude of these forces is measured by the zeta potential, which is: where: ... intermolecular, or van der Waals, attraction. Coagulants can be used to reduce the electrostatic repulsive ... In water treatment two main types of coagulants are used i.e. aluminum and iron salts. Type of coagulant formula most common
Exercise 5 DETERMINATION OF ADSORPTION ISOTHERM …
www.fpharm.uniba.skthe surface only through Van der Waals (weak intermolecular) interactions, which are also responsible for the non -ideal behaviour of real gases. Chemisorption is a type of adsorption whereby a molecule adheres to a surface through the formation of a chemical bond, as opposed to the Van der Waals forces which cause physisorption.
NATIONAL SENIOR CERTIFICATE/ NASIONALE …
ecexams.co.zaintermolecular forces/Van der Waals forces/dipoledipole forces between - molecules in PF. 3. Die . ioniese bindings. tussen deeltjies . in A. ℓ. F. 3 is sterker asdie
Intermolecular and Ionic Forces - web.gccaz.edu
web.gccaz.eduIntermolecular and Ionic Forces ... general, intermolecular forces are much weaker than the ionic and covalent bonds that hold together the atoms and ions in a compound. ... compared in this lab. The alkane used in this activity is pentane, CH 3 CH 2 CH 2 CH 2 CH 3. Alcohols are
Similar queries
Types of Intermolecular Forces, Everett Community College, Support, Intermolecular forces, Forces, Ionic, ACADEMIC YEAR 2020-2021, Chapter, Liquids, STATES OF MATTER, Inter molecular forces, INTERMOLECULAR FORCES Intermolecular forces, IMF Intermolecular Forces Worksheet, IMF – Intermolecular Forces Worksheet, Ionic compound, Intermolecular, Slide 1 Intermolecular Forces, Chapter 11 - Intermolecular Forces, Liquids and, Laboratory: Intermolecular Forces IMF, Drops on a penny, Chapter 11, The Chemistry of Food Colorings, American Chemical, Rate of Dissolving, 11 CHAPTER, Lecture 5: Coagulation and Flocculation, Types, Exercise 5 DETERMINATION OF ADSORPTION ISOTHERM, Intermolecular and Ionic Forces, Covalent