Search results with tag "Consort 2010"
Declaración CONSORT 2010 ... - consort-statement.org
www.consort-statement.orgTabla 1 CONSORT 2010. Lista de comprobacio´n de la informacio´n que hay que incluir al comunicar un ensayo clı´nico aleatorizado* Seccio´n/tema I´tem n8 I´tem de la lista de comprobacio´n Informado en
Item Reported No Checklist item on page No - Consort 2010
www.consort-statement.org*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and ...
The TIDieR (Template for Intervention Des cription and ...
www.equator-network.orgTIDieR checklist should be used in conjunction with the CONSORT statement (see www.consort‐statement.org) as an extension of Item 5 of the CONSORT 2010 Statement. When a clinical trial protocol is being reported, the TIDieR checklist should be used in conjunction with the SPIRIT statement as an extension of Item 11 of the SPIRIT 2013
Consort 2010 Statement
www.consort-statement.orghere the result of that process, CONSORT 2010. intent of consort 2010 The CONSORT 2010 Statement is this paper including the 25 item checklist in the table and the flow diagram. It pro‑ vides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials.
CONSORT 2010 lista de comprobación de ... - CONSORT …
www.consort-statement.orgCONSORT 2010 lista de comprobación de la información Pagina nº 1 CONSORT 2010 lista de comprobación de la información que hay que incluir al comunicar un ensayo clínico aleatorizado * Sección/tema Item nº Ítem de la lista de comprobación Informado en pagina nº Título y resumen 1a Identificado como un ensayo aleatorizado en el título
Consort 2010 Explanation and ... - CONSORT Statement
www.consort-statement.orgCONSORT statement and its accompanying explanation and elaboration document. The revised checklist is shown in table 1 and the flow diagram, not revised, in fig 1.52‑54 the consort 2010 statement: explanation and elaboration During the 2001 CONSORT revision, it became clear that explanation and elaboration of the principles underlying
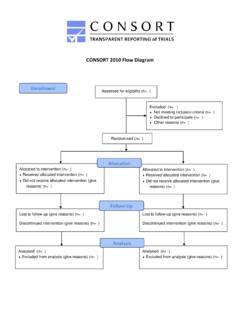
CONSORT 2010 Flow Diagram - CONSORT Statement
www.consort-statement.orgCONSORT 2010 Flow Diagram Assessed for eligibility (n= ) Excluded (n= ) Not meeting inclusion criteria (n= ) Declined to participate (n= ) Other reasons (n= ) Analysed (n= ) Excluded from analysis (give reasons) (n= ) Lost to follow-up (give reasons) (n= ) …
CONSORT CHECKLIST - JAMA
jamanetwork.comCONSORT CHECKLIST Table. CONSORT 2010 Checklist of Information to Include When Reporting a Randomized Triala Section and Topic Item No. Checklist Item Reported on Page No. Title and abstract 1a Identification as a randomized trial in the title 1b Structured summary of trial design, methods, results, and conclusions (for specific guidance see ...