Search results with tag "Atomic masses"
Example Exercise 9.1 Atomic Mass and Avogadro’s Number
www.austincc.eduWe begin by finding the atomic mass of each element in the periodic table. The molar mass equals the sum of the atomic masses expressed in g/mol. (a) The atomic mass of Ag is 107.87 amu, and the molar mass of silver equals 107.87 g/mol. (b) The sum of the atomic masses for NH. 3 is 14.01 amu + 3(1.01)amu = 17.04 amu. The molar mass of ammonia
Average Atomic Mass
mrsaultclassroom.weebly.comof atomic mass (and for unstable isotopes, radioactivity). Therefore, the periodic table lists a weighted average atomic mass for each element. In order to calculate this quantity, the natural abundance and atomic mass of each isotope must be provided. •N16. Consider the individual atomic masses for magnesium isotopes given in Model 2. a.
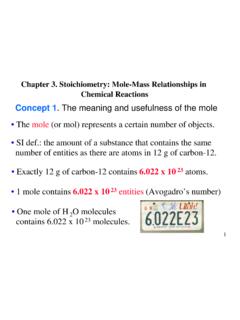
Chapter 3. Stoichiometry: Mole-Mass Relationships in ...
www.uh.edu• Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. • Molecular mass H 2 O = (2 x atomic mass of H) + atomic mass of O = 2(1.008 amu) + 16.00 amu = 18.02 amu • So, one mole of water (6.022 x 10 23 molecules) has a mass of 18.02 g. • Molar mass of NaCl = atomic mass of Na (22.99 amu) +
Nuclear Masses and Mass Excess: Q values for Nuclear …
www.astro.princeton.edumass of one 4He atom is about 0.71% less than the combined rest masses of four hydrogen atoms (note that the electrons are included in the atomic masses here). The difference, or about 26.7MeV, is released as heat, except for ≈ 0.6MeV worth of neutrinos (in the ppchain). There are two paths from 41Hto 4He: the pp cycle, which predominates in
CHAPTER : 1 SOME BASIC CONCEPTS OF CHEMISTRY 1 mark …
pue.kar.nic.in15. What is atomic mass unit? Ans: Atomic mass unit is defined as a mass exactly equal to 1/12 th the mass of one carbon 12 atom 16. What is the value of 1 a.m.u ? Ans: 1 a.m.u = 1.66056 x 10-24 g 17. Define molecular mass Ans: Molecular mass is the sum of atomic masses of the elements present in a molecule. 18. What is molar mass in gms?
Chapter 3. Chemical Stoichiometry 1. Atomic Masses ...
cribme.comChapter 3. Chemical Stoichiometry 1. Atomic Masses (section 2.5) ... Molarity and Solution Stoichiometry (Section 3.5) Molarity (M) is used to describe solution concentration because it is very easy to determine _____. Molarity = This quickly gives us the number of moles of solute: ...
Atomic mass is based on a relative scale and the mass of ...
www.westfield.ma.eduatomic masses stays the same. Since atoms of C are so small, we could place enough of them on a balance so that the mass would be 12.01 g. The same could be done with W; that is, 183.9 g of W could be placed on a balance. mass of W = 183.9 g = 15.31 mass of C 12.01 g 1 Since the ratio of the masses is the same, each sample contains 28