Example: barber
Search results with tag "Enthalpies of formation"
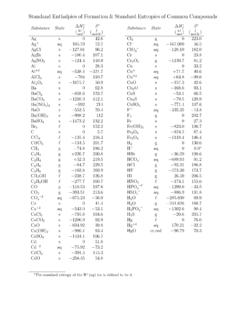
Standand Enthalpies of Formation & Standard Entropies of ...
www.mrbigler.comStandand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State ∆H f S (kJmol) (Jmol·K) Ag s 0 42.6 Ag+ aq 105.79 72.7 AgCl s −127.01 96.2
© www.CHEMSHEETS.co.uk 10-Mar-2016 Chemsheets A2 …
mchem.weebly.com1) Calculations involving enthalpies of formation (“Type 1 questions”) If the enthalpy of formation for the reactants and products in a reaction are known, the overall enthalpy change is easy to calculate. H = [SUM of f H products] – [SUM f H reactants] Remember that f H of all elements in their standard …