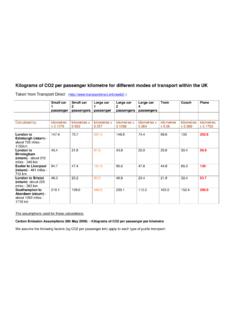
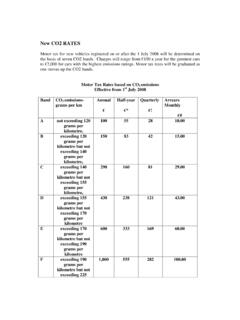
Transcription of CO2 Reduction Catalysts and Catalysis
1 1. CO2 Reduction Catalysts and Catalysis CHEM 462. Kristina Goldstein Soomin Park 2. Why does CO2 matter? (accessed 11/1/14). 3. Why does CO2 matter? (accessed 11/1/1. 4. Outline Introduction Catalysis Historical Background Catalyst and Kinetic Energy Catalytic Cycle CO2 Reduction Water Gas Shift Reaction Introducing CO2 Reduction Catalysts Methods Synthesis of Catalysts and Chemical Environments CV and Theory IR-SEC. Results Smieja Franco Riplinger Fischer-Tropsch Conclusions 5. Catalytic Power (history). J ns Jakob Berzelius (1835, Swedish chemist). Wilhelm Ostwald (1909 Nobel Prize in Chemistry). 20th Century ns_Jacob_Berzelius#mediaviewer/File:J (accessed 11/1/1. #mediaviewer/ (accessed 11/1/14). 6. Catalyst Rate of Reaction Pathway Activation Energy(Ea). Equilibrium #mediaviewer/ (accessed 11/1/14).))
2 7. Catalyst Rate of Reaction Pathway Activation Energy(Ea). Equilibrium (Keq). #mediaviewer/ (accessed 11/1/14). 8. Catalytic Cycle #mediaviewer/ (accessed 11/1/14). 9. CO2 Reduction (1958). Chemical Formula Name # of articles HCO2H formic acid 5. HCHO formaldehyde 6. CH3OH methanol 7. CH4 methane 2. CO carbon monoxide many J. Chem. Educ., 1958, 35 (9), 446-449. 10. CO2 Reduction CO2 + 2 H+ + 2 e- CO + H2O. (accessed 11/1/14). 11. Green Chemistry with Carbon Dioxide 12. Water Gas Shift Reaction (WGSR). CO2 + H2 = H2O + CO. 13. Water Gas Shift Reaction (WGSR). CO2 + H2 = H2O + CO. 14. Water Gas Shift Reaction (WGSR). 15. CO2 Reduction Catalytic Hydrogenation Complex Metal Hydrides Electrochemical Reduction Photocatalysis Biological Reduction - Carbon Monoxide DeHydrogenase: CODH. Chiang et al. Inorg.
3 Chem., 2005, 44, 9007-9016; J. Chem. Educ., 1958, 35 (9), 446-449. 16. Electrochemical Reduction TiO2 films MoS2 flakes Fe-porphyrin _dioxide#mediaviewer/ (accessed 11/1/14); M. Asadi, Nat. Commun., 2014, 5; 17. Transition Metals Complexes C. Riplinger at el, 2014, :2048/doi/ 18. Photocatalysis B. A. Parkinson, P. F. Weaver, Nature, 1984, 309, 148-149. 19. Biological Reduction Carbon Monoxide DeHydrogenase (CODH). 20. Synthesis of Catalysts Smieja Study Riplinger Study M=Re (white crystal), (yellow). Mn (orange crystal). (white). Smieja et al. Inorg. Chem. 2013, 52, 2484-2491; Riplinger et al. J. Am. Chem. Soc. 2014 (Just Accepted Manuscript). 21. Synthesis Continued Franco Catalyst (yellow). Why use a proton-assisted process? Sustained Catalysis in homogeneous solution even in the absence of Br nsted acids Reduce the large overpotentials required for multi-electron Catalysis , specifically during protonation Long durability in solution and high selective conversion of CO2 into CO at relatively low potentials Franco et al.
4 Chem. Commun., 2014, 00, 1-3; Chiang et al. Inorg. Chem., 2005, 44, 9007-9016. 22. Chemical Environments Addition of protons through a variety of acids (H2O, TFE, MeOH). Changes to atmospheric environment Exposure to CO2. Inert conditions (N2, Ar). Addition of different metals to ligands (Mn, Re, Pd, Rh, Ni, Fe, Co). 23. Cyclic Voltammetry What is Cyclic Voltammetry? Provides insights into both the kinetic and thermodynamic details of many chemical systems via redox reactions Includes a working electrode, reference electrode, counter electrode, and supporting electrolyte Types: homogenous and heterogeneous Dependent on scan rate, concentration and pH. Marken et al. Electroanalytical Methods, 2010, 2, 57-105. 24. Theory Behind CV. Rate of the homogeneous electron transfer step can be determined based on the measurement of peak potential and peak current data as a function of scan rate Reversibility determined by ratio between anodic and cathodic, peak current densities Internal standard consists of ferrocene, because it is completely reversible!
5 Counter electrode applies current to system to keep voltage constant as the chemical system absorbs electrons Ohm's Law: V=IR. Position of electrodes matter due to effects of IR(ohmic) drop Marken et al. Electroanalytical Methods, 2010, 2, 57-105. 25. Infrared-Spectroelectrochemistry (IR-SEC). A compliment to cyclic voltammetry Resolves the ambiguity of voltammetric data due to equilibrium of fast/slow steps on a voltammetric timescale in both the forward and backward direction Combines the thermodynamic data obtained from IR and the kinetic data obtained from CV. Marken et al. Electroanalytical Methods, 2010, 2, 57-105; Smieja et al. Inorg. Chem. 2013, 52, 2484-2491. 26. Smieja Results Increased response as more equivalents of acid were added to the CV cell The stronger the acid, the stronger the catalytic response Activated catalytic species: [Mn(bpy-tBu)(CO)3]- Mn(bpy-tBu)(CO)3 Br in MeCN under an Ar atmosphere Less overpotential than its Re counterpart 23.
6 Franco Catalyst Contains two acidic OH groups close to the metal centre, which showed a sustained catalytic response in homogeneous solution in the absence of acids Under a CO2 atmosphere in anhydrous MeCN. Partial stabilization of intermediate due to interaction between OH-Br and O-N(bpy). 2-/3- Reduction wave due to formation of a metal hydride Protons required for the catalytic process are supposed to be supplied by the Hofmann degradation of the supporting electrolyte, or hydrogen abstraction from the solvent Formation of HCOOH and CO confirm the presence of competing pathways and the hypothesis of hydride formation Franco et al. Chem. Commun., 2014, 00, 1-3. 24. Catalytic Activation Riplinger et al. J. Am. Chem. Soc. 2014. 25. CO/H2 Generation Riplinger et al. J. Am. Chem. Soc. 2014. Fischer-Tropsch Process Originated from need of liquid fuels by Germany during World War II.
7 Syngas from Water Shift Gas Reaction to Hydrocarbons Involves CO activation, C-C coupling, hydrogenation and desorption of the hydrocarbon product hydrogen mediated catalytic reductive polymerization of carbon monoxide . Atomic details are still unclear for these steps Exothermic reaction, increasing heat release with longer hydrocarbon chains Different metal centers contribute to different hydrocarbon formations Interest motivated by economic viability, environmental and energy security concerns Olusola et al. RSC Adv., 2012, 2, 7347 7366. 27. Conclusions Increasing interest in the industrial and academic communities A way to use a harmful greenhouse gas for synthesis of industrial compounds (CH4, CO, CH2O, Syngas). Multiple studies Purification and concentration of CO2 from the atmosphere to use the harmful levels of this greenhouse gas for energy storage Continue the approach to local proton sources on chelate ligands and mimicking naturally occurring catalytic systems Application of a solar cell to power Catalysis , and separation of byproducts 28.
8 Questions ? (accessed 2 NOV2014)