Transcription of guide to interpreting irrigation water analysis
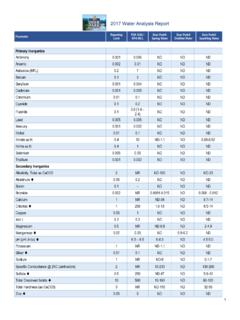
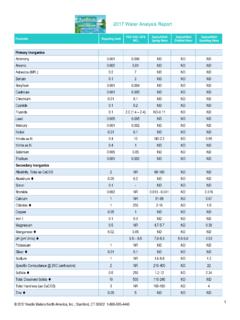
1 Spectrum Analytic, Analytic, Inc. Soil analysis 1087 Jamison Road 1-800-321-1562 Plant analysis PO Box 639 (740) 335-1562 Fertilizer analysis Washington , Ohio 43160 Fax: (740) 335-1104 Manure analysis guide TO interpreting irrigation water analysis 2 Table of Contents 2 water Salinity ..pg 3 water pH, Alkalinity, Bicarbonates and 4 Sodium Adsorption Ratio (SAR)..pg 6 Individual Elements or 8 Appendix A: Relative Soil Salt Tolerances of Agricultural and Horticultural 10 Deciduous 12 Coniferous 13 14 15 15 Grasses and Other Ground 16 Appendix B: Calculating Gypsum 17 Appendix C: Leaching 18 Appendix D: Acidification Procedures to Neutralize water 19 Appendix E.
2 Boron 20 Introduction While a few aspects of irrigation water quality have a direct impact on plants, the primary goal of water analysis is to judge the effect of the water on the soil, and ultimately on the plants grown on the soil. As such, much of the interpretation of the water analysis is based on a prediction of the consequences for the soil. The interpretation of the test results is, in many cases, dependant on the intended use of the water . Some plant species and production systems may have much different requirements or tolerances.
3 The interpretation guide lists some of these conditions and will help you evaluate your results. 3 water Salinity water salinity is a measure of the total dissolved salts. Saline water poses several hazards: As the salinity of the soil is increased by the use of water containing appreciable soluble salts, plants have increasing difficulty absorbing water . A primary cause of water salinity is excess sodium (Na). As Na accumulates in the soil it can compete with other nutrients for uptake by the plants and may become directly toxic.
4 Excess sodium in natural soil can lead to the loss of soil structure, causing the loss of soil permeability and leading to poor plant growth. water with too little dissolved salts can also be a problem in that it may lead to soil permeability problems. Where irrigation is applied by overhead sprinklers, excess water salinity can lead to foliage damage. SALINITY HAZARD LEVELS* Application Units None Increasing Significant High Severe All seedlings mmho/cm < > Container plants mmho/cm < > Nurseries** mmho/cm < > Field crops mmho/cm < > Hydroponics Field crops mmho/cm < > Soil Permeability** mmho/cm > < * See appendix A for more specific information ** Some common salt sensitive plants include Contoeaster horizontalis, Photina fraseri, Ilex cornuta, Vinca minor, Hibiscus rosasinensis, Nanadina domestica.
5 Azalea, Bardenia, and Limonium perezii. This list is not comprehensive ** The University of California has found that irrigation with exceptionally pure water has caused soil permeability problems with some vineyard soils in Eastern CA 4 water pH, Alkalinity, Bicarbonates, and Carbonates water pH The pH is a measurement of the relative acidity or basicity of the water . The pH range is from 0 to 14. Values from 0 to are acidic and those from to 14 are basic or alkaline, with being neutral. The pH scale is logarithmic, meaning that a change of unit is a ten-fold change in either acidity or basicity.
6 Therefore, changes of less than unit may be significant. This characteristic of the water has a significant influence on other characteristics or reactions in the soil and water , as well as the way plants perform. A water pH between and is normally considered to be the most desirable for irrigation . When the pH is outside of this range, it indicates that special actions may need to be taken to improve crop performance. Alkalinity This indicates the ability of the water to increase the pH of the soil or growing media, and the buffering power (resistance to change) of the water itself.
7 In other words, the ability of the water to act as a liming agent. Alkalinity is defined as the combined effect of bicarbonates (HCO3-) plus the carbonates (CO3--). High alkalinity indicates that the water will tend to increase the pH of the soil or growing media, possibly to a point that is detrimental to plant growth. Low alkalinity could also be a problem in some situations. This is because many fertilizers are acid-forming and could, over time, make the soil too acid for some plant. If the water is also somewhat acidic, the process would be accelerated.
8 Another aspect of alkalinity is its potential effect on sodium (Na-). Soil or artificial growing media irrigated with alkaline water may, upon drying, cause an excess of available sodium. Several potential problems could result. The excess available sodium could become directly toxic to some plants The salinity of the soil could be increased to the point that plant growth is damaged. Excess sodium could damage the structure of natural soil to the point that air and water infiltration are prevented, and root growth is restricted.
9 Among the components of water alkalinity, bicarbonates are normally the most significant concern. Typically, bicarbonates become an increasing concern as the water increases from a pH of to However, bicarbonates can be found in water of lower pH. Carbonates become a significant factor as the water pH increases beyond and are a dominant factor when the pH exceeds about High levels of bicarbonates can be directly toxic to some plant species. Bicarbonate levels above me/l (200 ppm) will cause lime (calcium and magnesium carbonate) to be deposited on foliage when irrigated with overhead sprinklers.
10 This may be undesirable for ornamental plants. Similar levels of bicarbonates may also cause lime deposits to form on roots, which can be especially damaging to many tree species. High water alkalinity can be corrected with acid injection (see appendix D). Danger from high alkalinity is governed in part by the volume of soil or artificial media involved. For example, greenhouse transplant production (plugs) have very little soil media and are less tolerant of a given alkalinity level than most other container production systems.