Ideal Gas Law Worksheet PV = nRT - New Providence School ...
1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters, what is the temperature? 2) If I have an unknown quantity of gas at a pressure of 1.2 atm, a volume of 31 liters, and a temperature of 87 0C, how many moles of gas do I have? 3) If I contain 3 moles of gas in a container with a volume of 60 liters and at a
Tags:
Information
Domain:
Source:
Link to this page:
Please notify us if you found a problem with this document:
Documents from same domain
Answers – Naming Chemical Compounds
www.npsd.k12.nj.usIonic/Covalent Compound Naming Solutions . For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately.
Chemical, Compound, Answers, Naming, Covalent, Covalent compound naming, Answers naming chemical compounds
Balancing Chemical Equations Using Models
www.npsd.k12.nj.usBalancing Chemical Equations Using Models Objective Use models as a means for balancing chemical equations. Materials . For each group of 4 students: ... Before Balancing After Balancing Before Balancing After Balancing Na 1 Na 1 O 1 O 1 H 2 H 2 Cl 1 Cl 1 . 6. ...
Using, Model, Chemical, Equations, Balancing, Balancing chemical equations, Balancing chemical equations using models
Introduction to Ionic Bonds I. Ionic Bonds III. Metallic ...
www.npsd.k12.nj.uscovalent bonding is found in nonmetallic elements and in nonmetallic compounds. Covalent bonds are intramolecular forces; that is, they are inside the molecule and hold the atoms together to make the molecule. Covalent bonds are strong bonds and it is difficult and requires a …
Chapter 9, Chemical Bonding i: Basic Concepts
www.npsd.k12.nj.usBonds of this type are described as nonpolar covalent bonds, or pure covalent bonds. In bonds involving different atoms, the electronegativity difference will …
Basics, Chemical, Concept, Bond, Bonding, Basic concept, Covalent, Covalent bonds, Chemical bonding i
Evaluating Joseph Stalin - New Providence School District
www.npsd.k12.nj.usEvaluating Joseph Stalin (Adapted from Document-Based Assessment for Global History, Walch Education) Historical Context: Joseph Stalin is one of the most controversial leaders in world history. Between 1928 and 1941 he . transformed the Soviet Union into a modern superpower. His rule is characterized by collectivized
The Alien Periodic Table - New Providence School District
www.npsd.k12.nj.us1 Alien Periodic Table Problem: Imagine that the inhabitants of another planet send a message to Earth that contains information about 30 elements. However, the message contains different names and symbols than those used on Earth.
ADAzg LoJw, DX~CIEL SINGER, A~YD JESS WINFIELD ACT ONE
www.npsd.k12.nj.usfeatures a book: The Complete Works of William Shakespeare. After a beat, DANIEL enters from the wings, ostensibly a house manager. He wears a watch.] DANIEL [Ratherserious.] Hello, and welcome to this performance of The Complete Works of William Shakespeare (abridged). I
Rudiments - New Providence School District
www.npsd.k12.nj.usRUDIMENTS R Single Stroke Roll LRLR LRL R Double Stroke Roll RLLR RLL R Paradiddle LRRL RLL
“The Myth of Sisyphus” Name Humanities Date
www.npsd.k12.nj.us“The Myth of Sisyphus” Name Humanities Date . 1. What does Camus mean when he says (at marker 1) “His scorn of the gods, his hatred
Date, Name, Myths, Humanities, Sisyphus, The myth of sisyphus name humanities date
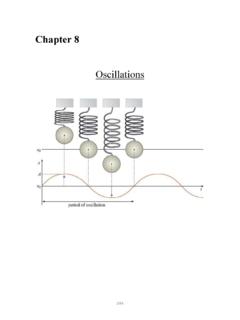
Chapter8 Oscillations
www.npsd.k12.nj.usQuestions 11-12 A sphere of mass m1, which is attached to a spring, is displaced downward from its equilibrium position as shown above left and released from rest. A sphere of mass m2, which is suspended from a string of length L, is displaced to the right as shown above right and released from rest so that it swings as a simple pendulum with small amplitude.
Related documents
Gas Laws Worksheet - New Providence School District
www.npsd.k12.nj.us2. If 10.0 liters of oxygen at STP are heated to 512 °C, what will be the new volume of gas if the pressure is also increased to 1520.0 mm of mercury? 3. A gas is heated from 263.0 K to 298.0 K and the volume is increased from 24.0 liters to 35.0 liters by moving a large piston within a cylinder. If the original pressure was 1.00 atm, what ...
Estimated water consumption in the world + Per appliance ...
wastewatergardens.com1 US Gallon = 3.7854 Liters 1 squ. foot = 0.09290 Square meter Per person per day Gallons Liters Unit Comments and Sources TOILETS: Average of 5 flushes / day / person TOILET / Sitting flush toilet Minimum 6 Per Flush Average toilets after 2000 but each toilet must be checked 30 Per day Maximum 23 Per Flush Older toilets 115 Per day TOILET ...
THE LIFE CYCLE OF A JEAN - Levi Strauss & Co.
www.levistrauss.com3,781 liters… Eutrophication: 48.9 g PO. 4-e… • 69 miles driven by the average US car • 246 hours of TV on a plasma big-screen 3 days worth of one US household’s total water needs . The total amount of phosphorous found in 1,700 tomatoes . The entire lifecycle of one pair of Levi’s® 501® jeans equates to:
IS 1172 (1993): Code of Basic Requirements for Water ...
dasta.inI IS1172:1993 Indian Standard CODE OF BASIC REQUIREMENTS FOR WATER SUPPLY, DRAINGE AND SANITATION ( Fourth Revision ) UDC 625 i/.3 : 006.76 BUREAU OF INDIAN STANDARDS
Basics, Code, Requirements, Water, Code of basic requirements for water
USEFUL WATER COMPARISONS
www.nswic.org.auNSWIC NEW SOUTH WALES IRRIGATORS’ COUNCIL PO Box R1437 Royal Exchange NSW 1225 Tel: 02 9251 8466 Fax: 02 9251 8477 [email protected] www.nswic.org.au
Statistics: Dairy cows - Compassion in World Farming
www.ciwf.org.ukPage 1 of 8 Updated 01.07.2012. Statistics: Dairy cows . Population & Production . World • There are over 264 million dairy cows worldwide, producing nearly 600 million tonnes of milk every year (source FAOstat – see table 1). • The global average for milk production is approximately 2,200 litres per cow (source FAOstat 2012). • The largest producer of milk is the USA producing over …
Grade 5 Mathematics Vocabulary Word Wall Cards Table of ...
www.doe.virginia.govVirginia Department of Education 2018 Grade 5 Mathematics Vocabulary – Card 2 Round Round 1.24 to the nearest tenth. 1.2 1.24 1.3
Virginia department of education, Virginia, Department, Education
The Children’s Hospital Children’s fluid management
www.ouh.nhs.ukOMI 4510P If you need an interpreter or need a document in another language, large print, Braille or audio version, please call 01865 221473 or email [email protected] Louiza Dale, Angela Downer, Paediatric Urology Nurse Specialists
Mole Calculation Worksheet - Brookside High School
www.sheffieldschools.org6) How many grams are in 11.9 moles of chromium? 7) How many moles are in 9.8 grams of calcium? 8) How many grams are in 238 moles of arsenic? Solve the following: 9) How many grams are in 4.5 moles of sodium fluoride, NaF? (molar mass of NaF is 22.99 + 19.00 = 41.99 g/ mole) 4.5 moles x 41.99 grams = 188.955 g NaF =