Transcription of Objectives Chemical Kinetics
1 Chemistry, by its very nature, is concerned with with well defined properties are convertedby Chemical reactions into other substances withdifferent properties. For any Chemical reaction, chemiststry to find out(a) the feasibility of a Chemical reaction which can bepredicted by thermodynamics ( as you know that areaction with G < 0, at constant temperature andpressure is feasible);(b) extent to which a reaction will proceed can bedetermined from Chemical equilibrium;(c) speed of a reaction time taken by a reaction toreach with feasibility and extent, it is equallyimportant to know the rate and the factors controllingthe rate of a Chemical reaction for its completeunderstanding.
2 For example, which parametersdetermine as to how rapidly food gets spoiled? Howto design a rapidly setting material for dental filling?Or what controls the rate at which fuel burns in anauto engine? All these questions can be answered bythe branch of chemistry, which deals with the studyof reaction rates and their mechanisms, calledchemical Kinetics . The word Kinetics is derived fromthe Greek word kinesis meaning tells only about the feasibility of areaction whereas Chemical Kinetics tells about the rateof a reaction. For example, thermodynamic dataindicate that diamond shall convert to graphite butin reality the conversion rate is so slow that the changeis not perceptible at all.
3 Therefore, most people thinkAfter studying this Unit, you will beable to define the average andinstantaneous rate of a reaction; express the rate of a reaction interms of change in concentrationof either of the reactants orproducts with time; distinguish between elementaryand complex reactions ; differentiate between themolecularity and order of areaction; define rate constant; discuss the dependence of rate ofreactions on concentration,temperature and catalyst; derive integrated rate equationsfor the zero and first orderreactions; determine the rate constants forzeroth and first order reactions ; describe collision Kinetics helps us to understand how Chemical KChemical KChemical KChemical KChemical KineineineineineticsticsticsticsticsUnit UnitUnitUnitUnit4 Chemical KChemical KChemical KChemical KChemical Kineineineineineticsticsticsticstics94 Chemistrythat diamond is forever.
4 Kinetic studies not only help us to determinethe speed or rate of a Chemical reaction but also describe theconditions by which the reaction rates can be altered. The factorssuch as concentration, temperature, pressure and catalyst affect therate of a reaction. At the macroscopic level, we are interested inamounts reacted or formed and the rates of their consumption orformation. At the molecular level, the reaction mechanisms involvingorientation and energy of molecules undergoing collisions,are this Unit, we shall be dealing with average and instantaneousrate of reaction and the factors affecting these. Some elementaryideas about the collision theory of reaction rates are also , in order to understand all these, let us first learn about thereaction reactions such as ionic reactions occur very fast, for example,precipitation of silver chloride occurs instantaneously by mixing ofaqueous solutions of silver nitrate and sodium chloride.
5 On the otherhand, some reactions are very slow, for example, rusting of iron inthe presence of air and moisture. Also there are reactions like inversionof cane sugar and hydrolysis of starch, which proceed with a moderatespeed. Can you think of more examples from each category?You must be knowing that speed of an automobile is expressed interms of change in the position or distance covered by it in a certainperiod of time. Similarly, the speed of a reaction or the rate of areaction can be defined as the change in concentration of a reactantor product in unit time. To be more specific, it can be expressed interms of:(i) the rate of decrease in concentration of any one of thereactants, or(ii)the rate of increase in concentration of any one of the a hypothetical reaction, assuming that the volume of thesystem remains POne mole of the reactant R produces one mole of the product P.
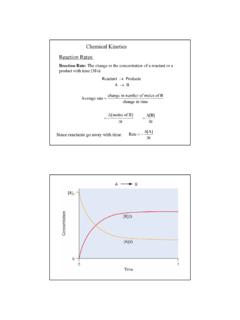
6 If[R]1 and [P]1 are the concentrations of R and P respectively at time t1and [R]2 and [P]2 are their concentrations at time t2 then, t=t2 t1 [R] = [R]2 [R]1 [P] = [P]2 [P]1 The square brackets in the above expressions are used to expressmolar of disappearance of R Decrease in concentration of RR=Time takent ( ) of aRate of aRate of aRate of aRate of aChemicalChemicalChemicalChemicalChemica lReactionReactionReactionReactionReactio n95 Chemical KineticsRate of appearance of P Increase in concentration of PP=Time takent ( )Since, [R] is a negative quantity (as concentration of reactants isdecreasing), it is multiplied with 1 to make the rate of the reaction apositive ( ) and ( ) given above represent the average rate ofa reaction, rate depends upon the change in concentration of reactantsor products and the time taken for that change to occur (Fig.)
7 Fig. : Instantaneous and average rate of a reactionUnits of rate of a reactionFrom equations ( ) and ( ), it is clear that units of rate areconcentration time 1. For example, if concentration is in mol L 1 andtime is in seconds then the units will be mol L-1s 1. However, in gaseousreactions, when the concentration of gases is expressed in terms of theirpartial pressures, then the units of the rate equation will be atm s the concentrations of C4H9Cl (butyl chloride) at different times givenbelow, calculate the average rate of the reaction:C4H9Cl + H2O C4H9OH + HClduring different intervals of [C4H9Cl]/mol L can determine the difference in concentration over different intervalsof time and thus determine the average rate by dividing [R] by t(Table ).
8 { }Example can be seen (Table ) that the average rate falls from 0-4 mol L-1s-1to 10-4 mol L-1s-1. However, average rate cannot be used to predictthe rate of a reaction at a particular instant as it would be constant for thetime interval for which it is calculated. So, to express the rate at a particularmoment of time we determine the instantaneous rate. It is obtained whenwe consider the average rate at the smallest time interval say dt ( when t approaches zero). Hence, mathematically for an infinitesimally smalldt instantaneous rate is given by avRPrtt( )As t 0or instddRPddrtt Table : Average rates of hydrolysis of butyl chloride[C4H9CI]t1 /[C4H9CI]t2 /t1/st2/srav 104/mol L 1s 1 mol L 1mol L 1 = 21449492 1ttCHCl CHCl /t rate ofhydrolysis of butylchloride(C4H9Cl)97 Chemical KineticsIt can be determined graphically by drawing a tangent at time t oneither of the curves for concentration of R and P vs time t and calculatingits slope (Fig.)
9 So in problem , rinst at 600s for example, can becalculated by plotting concentration of butyl chloride as a function oftime. A tangent is drawn that touches the curve at t = 600 s (Fig. ).The slope of this tangent gives the instantaneous , rinst at 600 s = - 400 s mol L 1 = 10 5 mol L 1s 1At t = 250 srinst = 10 4 mol L 1s 1 t = 350 srinst = 10 4 mol L 1s 1 t = 450 srinst = 10 5 mol L 1s 1 Now consider a reactionHg(l) + Cl2 (g) HgCl2(s)Where stoichiometric coefficients of the reactants and products aresame, then rate of the reaction is given as 22 HgClHgClRate of reaction = ttt , rate of disappearance of any of the reactants is same as the rateof appearance of the products.
10 But in the following reaction, two moles ofHI decompose to produce one mole each of H2 and I2,2HI(g) H2(g) + I2(g)For expressing the rate of such a reaction where stoichiometriccoefficients of reactants or products are not equal to one, rate ofdisappearance of any of the reactants or the rate of appearance ofproducts is divided by their respective stoichiometric coefficients. Sincerate of consumption of HI is twice the rate of formation of H2 or I2, tomake them equal, the term [HI] is divided by 2. The rate of this reactionis given byRate of reaction 22HI1HI2ttt Similarly, for the reaction5 Br- (aq) + BrO3 (aq) + 6 H+ (aq) 3 Br2 (aq) + 3 H2O (l) 322 BrOBrH O1111 BrHRate5633tt ttt For a gaseous reaction at constant temperature, concentration isdirectly proportional to the partial pressure of a species and hence, ratecan also be expressed as rate of change in partial pressure of the reactantor the QuestionsIntext QuestionsIntext QuestionsIntext QuestionsIntext the reaction R P, the concentration of a reactant changes from in 25 minutes.