Transcription of Page 1 of 4 Paclitaxel Albumin (Abraxane®) and …
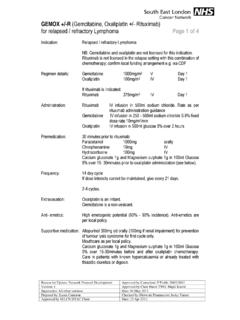
1 Page 1 of 4 Reason for Update: addition to NCDF Approved by Consultant: Paul Ross Version: 1 Date: 04/04/14 Supersedes: Nil Checked by (Area Team Cancer Pharmacist): Jacky Turner Prepared by: Lisa Yuen 25/03/2014 Date: 09/04/14 Paclitaxel Albumin (Abraxane ) and gemcitabine in Pancreatic Cancer Indication: First line treatment for advanced adenocarcinoma of the pancreas National Cancer Drug Fund criteria to be met: Histologically or cytologically confirmed adenocarcinoma of the pancreas Stage IV disease (patients with locally advanced disease are ineligible) PS 0 or 1 No previous chemotherapy for advanced disease No previous chemotherapy for early disease unless given as a radiation sensitiser at least 6 months previously Ensure funding has been approved prior to prescribing Regimen details: Paclitaxel Albumin (Abraxane) 125mg/m2 IV Day 1, 8 and 15 gemcitabine 1000mg/m2 IV Day 1, 8 and 15 Administration: Paclitaxel Albumin will be supplied as a 5mg/ml solution in an empty 250ml infusion bag.
2 The dose should be delivered IV over 30 minutes, do not use an in-line filter. Followed by gemcitabine in 250-500ml Sodium Chloride IV over 30 minutes Frequency: Days 1, 8 and 15, every 28 days Extravasation: Paclitaxel Albumin (Abraxane) vesicant gemcitabine non-vesicant Anti-emetics: Low emetogenicity Follow local anti-emetic policy. Supportive medication: Routine pre-medication not required. Regular investigations: FBC D1, D8, D15 U&Es D1 LFTs D1 ECHO/MUGA If clinically indicated (see comments) Toxicities: Myelosuppression, infection, alopecia, neuropathy, fatigue, myalgia, arthalgia, nausea, vomiting, diarrhoea, constipation, anaemia, elevation of liver transaminases (AST/ALT) and ALP, mucositis, anorexia, skin reactions, flu-like symptoms, peripheral oedema, proteinuria, haematuria, cardiotoxicity, eye problems, somnolence, nail changes, haemolytic uraemic syndrome, pulmonary effects, hypersensitivity reactions.
3 Page 2 of 4 Reason for Update: addition to NCDF Approved by Consultant: Paul Ross Version: 1 Date: 04/04/14 Supersedes: Nil Checked by (Area Team Cancer Pharmacist): Jacky Turner Prepared by: Lisa Yuen 25/03/2014 Date: 09/04/14 DOSE MODIFICATIONS Haematological Toxicity Dose level reductions for haematological toxicity Dose level Paclitaxel Albumin (Abraxane) dose gemcitabine dose Full dose 125mg/m2 1000mg/m2 1st dose reduction 100mg/m2 800mg/m2 2nd dose reduction 75mg/m2 600mg/m2 If further dose reduction required Discontinue Discontinue Prior to day 1 Neutrophils (x 109/L) Platelets (x 109/L) Dose modification and 100 Full dose < or < 100 Delay for 1 week.
4 Repeat FBC, if recovered to above these levels, resume treatment at full dose. Consider dose reduction for >1 delay. Prior to day 8 Neutrophils (x 109/L) Platelets (x 109/L) Dose modification and 75 Full dose - or 50 - 75 Reduce by 1 dose level < or < 75 Omit Prior to 15 Neutrophils (x 109/L) Platelets (x 109/L) Dose modification and 75 Give same dose as on day 8 OR if day 8 dose was omitted, reduce by 1 dose level - or 50 - 75 Reduce by 1 dose level from day 8 dose OR if day 8 dose was omitted reduce by 2 dose levels < or < 75 Omit Non-haematological toxicities Renal Impairment Abraxane - Studies in patients with impaired renal function have not been performed and insufficient data are currently available to recommend dose modifications in patients with renal impairment.
5 gemcitabine - Use with caution in patients with creatinine clearance < ; however, no specific dosing recommendations have been made. Page 3 of 4 Hepatic Impairment Bilirubin ALT/AST Abraxane dose < 2 x ULN < x ULN Give 100% dose 2 x ULN 9 x ULN Discuss with consultant and consider dose reduction > 5 x ULN and/or > 10 x ULN Has not been studied do not treat gemcitabine Administration of gemcitabine in patients with liver metastatases or a pre-existing medical history of hepatitis, alcoholism, or liver cirrhosis may lead to exacerbation of the underlying hepatic insufficiency. Use with caution in the presence of hepatic dysfunction: AST elevations do not seem to cause dose limiting toxicities If bilirubin >27 mol/L, initiate treatment with 800mg/m2 Dose modifications for other toxicities Abraxane dose gemcitabine dose Grade 3 or 4 Febrile Neutropenia Withhold until fever resolves and neutrophils recover to x 109/L, then reduce by 1 dose level Grade 3 or 4 Peripheral Neuropathy Withhold until resolves to Grade 1 then reduce by 1 dose level Continue gemcitabine treatment Grade 2 or 3 Cutaneous Toxicity Withhold until resolves to Grade 1 then reduce by 1 dose level Gastrointestinal Toxicity: Grade 3 mucositis or diarrhoea Withhold until resolves to Grade 1 then reduce by 1 dose level Comments: Hypersensitivity Hypersensitivity reactions are rare, however very rare events of anaphylaxis have been reported.
6 Availability of resuscitation equipment must be ensured as a standard precaution. If a hypersensitivity reaction occurs, treatment should be discontinued immediately and symptomatic treatment should be initiated. The patient should not be rechallenged. Haemolytic uraemic gemcitabine should be discontinued at the first signs of any evidence of syndrome microangiopathic haemolytic anaemia, such as rapidly falling haemoglobin with concomitant thrombocytopenia, elevation of serum bilirubin, serum creatinine, blood urea nitrogen, or LDH, which may indicate development of haemolytic uraemic syndrome. Renal failure may not be reversible, even with discontinuation of therapy, and dialysis may be required Cardiotoxicity Rare reports of congestive heart failure and left ventricular dysfunction have been observed in patients with underlying cardiac history or previous exposure to cardiotoxic Reason for Update: addition to NCDF Approved by Consultant: Paul Ross Version: 1 Date: 04/04/14 Supersedes: Nil Checked by (Area Team Cancer Pharmacist): Jacky Turner Prepared by: Lisa Yuen 25/03/2014 Date: 09/04/14 Page 4 of 4 Reason for Update: addition to NCDF Approved by Consultant: Paul Ross Version: 1 Date: 04/04/14 Supersedes: Nil Checked by (Area Team Cancer Pharmacist): Jacky Turner Prepared by: Lisa Yuen 25/03/2014 Date: 09/04/14 products such as anthracyclines.
7 Patients should be monitored for the occurrence of cardiac events. Pulmonary effects Pulmonary effects such as pulmonary oedema, interstitial pneumonitis or adult respiratory distress syndrome has been reported, treatment should be permanently discontinued and appropriate supportive measures initiated. Visual acuity Post-marketing experience has identified rare reports of reduced visual acuity due to cystoid macular oedema. Abraxane treatment should be discontinued. Sodium content Each ml of Paclitaxel Albumin (Abraxane) contains approximately of sodium, and each vial of gemcitabine 200mg contains of sodium. To be taken into consideration by patients on a controlled sodium diet. Drug interactions: No formal drug interaction studies have been performed The metabolism of Paclitaxel is catalysed, in part, by cytochrome P450 isoenzymes CYP2C8 and CYP3A4. Caution should be exercised when administering Paclitaxel concomitantly with medicines known to inhibit ( ketoconazole and other imidazole antifungals, erythromycin, fluoxetine, gemfibrozil, cimetidine, ritonavir, saquinavir, indinavir, and nelfinavir) or induce ( rifampicin, carbamazepine, phenytoin, efavirenz, nevirapine) either CYP2C8 or CYP3A4.
8 gemcitabine is a radiosensitizer -Warfarin: increased anticoagulant effect of warfarin References: Celgene Ltd. Summary of Product Characteristics: Abraxane 16/01/2014 Available at [Accessed 25/03/14] Sun Pharmaceuticals UK Ltd. Summary of product characteristics: gemcitabine 22/04/2013 Available at [Accessed 25/03/14] UCLH Dosage adjustment for cytotoxics in renal impairment. Jan 2009 UCLH Dosage adjustment for cytotoxics in hepatic impairment. Jan 2009 Von Hoff, , et al. Results of a randomized phase III trial (MPACT) of weekly nab- Paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas with PET and CA19-9 correlates. J Clin Oncol 31, 2013 (suppl; abstr 4005^) Von Hoff DD et al. Increased Survival in Pancreatic Cancer with nab- Paclitaxel plus gemcitabine N Engl J Med 2013;369:1691-703 National Cancer Drug Fund Decision Summary March 2014