Transcription of pH and Acid Base Indicators - Yosemite Community College ...
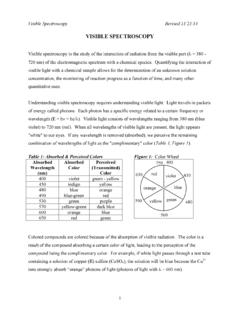
1 PH and acid base Indicators Page 1 pH and acid base Indicators Problem and Purpose: In this experiment you will learn to use both common Indicators and pH paper, assess their efficacy and test the pH of both common household substances and prepared buffered solutions . Subsequently, you will investigate the pH behavior of these substances as well as some weak bases. Introduction: An acid base indicator is a substance that changes color as the pH of a solution changes. [see pages 310 and 311 in your textbook]. There are countless numbers of different acid base Indicators , many of which can be extracted from common plants. Every indicator exhibits a different range of colors at different pH values. For example, the indicator phenolpthalein, which you used in the week 5 lab Titration , is colorless in solutions with a pH less than 8 and pink to deep red in solutions with a pH greater than 8.
2 Bromothymol blue is yellow in acidic solutions and blue in basic solutions . Of course, an indicator can be used to judge the pH of a solution only if the characteristic colors at various pH s are previously known. Some of the Indicators available to us in this Chem 10 laboratory are pictured below: Indicators work because they are weak acids which, when in solution, exist in equilibrium with their conjugate base . The acid and its conjugate base each have different colors; as the equilibrium shifts from one direction to the other, the color of the indicator solution also changes. Let s use bromothymol blue as an example; we ll represent the equilibrium the pertinent equation establishes as: pH and acid base Indicators Page 2 HB --> B + H+ yellow blue Indicators are generally large organic molecules with complicated formulas, so, for convenience, we ll abbreviate the indicator as HB.
3 Consistent with LeCh telier, if a solution containing the indicator becomes acidic ( , with the addition of H+), the equilibrium will shift to the left, and the solution will become yellow. If, on the other hand, we make the solution basic ( , if OH ions are added, which would reduce the hydronium concentration), the equilibrium will shift to the right and the solution will become blue . At intermediate pH values there will be a mixture of both HB and B , and the solution will take on a green color. Some Indicators exhibit only two colors and some exhibit a wide range. Each indicator must be individually studied to determine its behavior as a function of pH. pH paper consists of strips of filter paper which have been previously soaked in an indicator and subsequently dried. A drop of an unknown solution can be placed on the pH paper, and the resulting color compared to a chart.
4 By matching the color of the paper to a color on the chart, the pH of the solution can be determined. Procedure In this experiment you are to work in teams. Using both pH paper, Indicators from a selection of those available to Chem 10 and a natural indicator prepared freshly from red cabbage juice, follow the steps outlined below to determine the pH of each of these common household substances you have added to one slot in your spotting plate : Baking soda Vinegar Bleach Lemon juice Aspirin Antacid tablets Drain cleaner (Are your goggles on!) Household ammonia For solids, prepare a saturated solution by dissolving as much solid as possible in a small amount of water, then test the pH as indicated above. Part I Determining the pH Using pH Paper and Other Indicators Use the pH paper and at least three available Indicators to determine the pH of four of the household substances on the list above.
5 1. Touch a piece of pH paper to each of the household materials above contained in each slot in the spotting plate described above and note the pH from the scale; 2. Add a drop of an indicator of your choice to each of the indentations containing the household materials and note the colors; 3. Repeat step #2 immediately above with a different indicator . Record your results in table form in your lab notebook, , material/pH from pH paper/ indicator color. pH and acid base Indicators Page 3 Part II Determining the pH Behavior of Red Cabbage Juice Water 1. Set up eight small test tubes and add an equal amount of the cabbage water to each. 2. Add items from the list of household substances used in part I via eyedropper or pipette to the cabbage water until a color change is effected and observe the color of the resulting solutions .
6 The cabbage contains an indicator which changes color as the pH of the solution changes. 3. Use the pH paper to measure the pH of each solution, and record the color of the cabbage juice at those different pH s. As always, make certain to record all your results in tabular form in your lab book, , material, pH and resulting color of cabbage water. Part III Determining the pH Behavior of Buffer Tablets We have several containers of Coleman pH buffer tablets in a variety of pH levels available to our Chem 10 laboratories; see the photograph below: In 50 mL beakers, as a class prepare one solution per tablet [per directions given] except for the buffered pH tablet; thus, we will have six solutions of varying pH, one in each beaker. 1. Fill twelve indentations two from each of the six solutions prepared from the buffer tablets - in the spotting plate as described above for a total of 12 ; pH and acid base Indicators Page 4 2.
7 Touch a piece of pH paper to each of the six unique buffer solutions above contained in each slot in the spotting plate as described above and note the pH from the scale; 3. Use the two Indicators you chose to use in Section #1 above; put a drop of each into the six unique pH buffer solutions prepared above note the color of each of the six pHs for each indicator and record all twelve in table form into your notebook pH/ indicator /color. Analysis Prepare a neat table summarizing your results for the lab for each of the three sections above. Include the results for the pH of each of the household substances, ranked from lowest to highest pH, and then the corresponding color of the cabbage solution. For Part 3, list the pH and the color for each of the Indicators you choose to utilize.
8 As in previous write ups, include a thoughtful discussion of your results in the Analysis section of your lab report. Questions 1. Which of the household materials was the most acidic? What was the indicator color for the two Indicators for this? The most basic? What was the indicator color for the two Indicators for this? 2. What s the color or shade of color for acidic solutions with cabbage juice? What s the color of basic solutions in cabbage juice? 3. Which of the three Indicators you used has the widest variation with pH? 4. Would it be possible to use cabbage use as the basis for a type of pH paper?
9 Why? How would you prepare this paper?