Transcription of Preferred reporting items for systematic review and meta ...
1 Preferredreportingitemsfor systematicreviewandmeta-analysisprotocol s( prisma -P) 2015 :elaborationand explanationLarissaShamseer1, DavidMoher1, MikeClarke2, DavinaGhersi3, AlessandroLiberati(deceased)4,MarkPettic rew5, Paul Shekelle6, LesleyA Stewart7, the prisma -PGroup1 OttawaHospitalResearchInstituteand Universityof Ottawa,Canada;2 Queen sUniversityBelfast,Ireland;3 NationalHealthand MedicalResearchCouncil,Australia;4 Universityof Modena,Italy;5 LondonSchoolof Hygieneand TropicalMedicine,UK;6 SouthernCaliforniaEvidence-basedPractice Center,USA;7 Centrefor Reviewsand Dissemination,Universityof York,UKDedication.
2 The prisma -P2015initiativeis dedicatedto our colleagueAlessandroLiberati(1954 2012),who passedawaywhilePRISMA-P2015was underdevelopmentand whosecontributionsto this work systematicreviewsand meta-analysesallowfor planningand documentationof reviewmethods,act as a guardagainstarbitrarydecisionmakingdurin greviewconduct,enablereadersto assessforthe presenceof selectivereportingagainstcompletedreview s,and,whenmadepubliclyavailable,reducedu plicationof effortsand existenceof selectivereportingand excessiveduplicationof reviewson the sameor similartopicsis accumulatingand manycalls havebeenmadein supportofthe documentationand publicavailabilityof recentyearsto rectifytheseproblems,includingdevelopmen tof an internationalregisterfor prospectivereviews(PROSPERO)and launchof the first openaccessjournaldedicatedtothe exclusivepublicationof systematicreviewproducts,includingprotoc ols(BioMedCentral sSystematicReviews).
3 Furtheringtheseeffortsand buildingon the prisma (PreferredReportingItemsforSystema ticReviewsand Meta-analyses)guidelines,an internationalgroupof expertshas createda guidelineto improvethe transparency,accuracy,completeness,and frequencyof documentedsystematicreviewand meta-analysisprotocols prisma -P(for protocols) prisma -Pchecklistcontains17 itemsconsideredto be essentialand minimumcomponentsof a systematicreviewor prisma -P2015 Explanationand Elaborationpaperprovidesreaderswith a full understandingof and evidenceaboutthe necessityof eachitem as well as a modelexamplefrom an papershouldbe read togetherwith the assessorsare stronglyencouragedto makeuse of prisma -Pwhendraftingand a uniqueplacein helpform the basis for developingpracticeguidelinesand theyprovideinformationon gaps in knowledge,thus informationis relevantto stakeholdersacrossthe rigourand trustworthinessofsystematicreviewsis.
4 In large part, basedon the a prioriplanningand documentationof a methodicalapproachtoconduct(that is, a protocol).A systematicreviewprotocolis importantfor severalreasons:(1) it allowssystematicreviewersto plan carefullyand therebyanticipatepotentialproblems;(2) it allowsreviewersto explicitlydocumentwhat is plannedbeforethey start their review ,enablingothersto comparethe protocoland the completedreview(that is, to identifyselectivereporting),to replicatereviewmethodsif desired,and to judgethe validityof plannedmethods;(3) it preventsarbitrarydecisionmakingwith respectto inclusioncriteriaand extractionof data.
5 And (4) it may reduceduplicationof effortsand enhancecollaboration, as the CochraneandCampbellCollaborationsand the Agencyfor HealthcareResearchand Quality(AHRQ)regularlyrequireand ,outsideof such organizations,few protocolsare publishedin traditionaljournalsand most reportsofcompletedreviews(89%)do not mentionworkingfrom aprotocol1(2014updateunderway).Manyexper tshave calledfor improveddocumentationand availabilityof response,experts(someof whomare authorson thisdocument)launchedan international,prospectiveregisterforsyst ematicreviewprotocols(PROSPERO, ) throughthe Centrefor Reviewsand Disseminationat the Universityof York (UK)in February2011,in whichmorethan 5000 systematicreviewprotocolsfrom 69 countrieshavebeen registeredas of February2012,theCorrespondence to: L Shamseer personal use only: See rights and reprints.
6 349:g7647doi: (Published2 January2015)Page1 of 25 ResearchMethods& ReportingRESEARCHMETHODS& reporting first open accessjournalto exclusivelypublishsystematicreviewproduc tsincludingprotocols(BioMedCentral sSystematicReviews) was launched,in which142 protocolshave beenpublished(June2014).Outsideof selectsystematicrevieworganizations,litt le to no generalguidanceexistsfor of the most importantfunctionsof systematicreviewprotocolsis their role as a documentationof plannedreviewmethods,outcomes,and analysesthat can be comparedwith completedreviewsto detectwhetherunintendedandundocumentedch angeswere relatedto selectivereportingof outcomes(that is, whenreportingis relatedto thestatisticalsignificanceor directionof effectestimate)
7 Is aproblemin is a well documentedphenomenonin clinicaltrials,2-7and similarfindingsare startingto emergefor systematicreviews(see item 13 for fulldiscussion).8-10 Whenreviewersselectivelychoosewhichinfor mationto includein a reportbasedon the directionandsignificanceof findings,they risk biasingthe evidencebase onwhichhealthcaredecisionsand policiesare recenteffortsto increasethe documentationandavailabilityof reviewprotocols,the next logicalstep is thedevelopmentof a set of standardsthat shouldbe includedin well describedprotocolmay facilitateandenhancethe detectionof undocumentedchangesto reviewmethodology.
8 It also may allowreadersto gaugethe potentialimpactof such changesas well as selectivereportingofinformationon that end, a reportingguidelinefor systematicreviewprotocols,an extensionof the prisma (PreferredItemsforReportingSystema ticReviewsand Meta-analyses)statementhas been developedfor protocols( prisma -P)and is describedin detailin this prisma -PPRISMA-Pis intendedto guidethe developmentof protocolsof systematicreviewsand systematicreviewsthat are not evaluatingefficacy,authorsare encouragedto use prisma -Pbecauseofthe lack of the purposeof this guidance,we definea protocol,broadly,as a documentwrittenbeforethe start of a systematicreviewdescribingtherationalean d intendedpurposeof the review ,and the plannedmethodologicaland analyticalapproach(see box 1 forcomprehensivedefinitions).
9 prisma -Pis meantto be used primarilyby authorspreparingsystematicreviewprotocol sfor publication,publicconsumption,or is also intendedfor thosecommissioningandpotentiallyfundingr eviewsas a guidefor applicantson whatshouldthey shouldincludein their reviewprotocols,and as atool for peer reviewersto gaugewhethera also be helpfulfor journaleditorsand peer reviewersgaugingthe adequacyof reviewprotocolsfor list of stakeholdersto whomwebelievePRISMA-Pwill be usefulalongwith proposedbenefitsfor each groupis providedin table 1 .Developmentof prisma -PThe prisma -Pchecklistis basedon elementsfrom thePROSPERO register,11the prisma checklist,12 SPIRIT(StandardProtocolItems.)
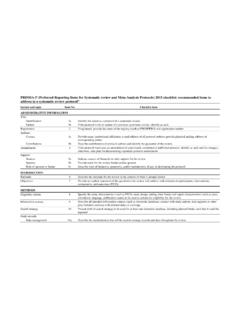
10 Recommendationsfor InterventionalTrials)checklistitems,13an d from the InstituteofMedicine sStandardsfor detaileddescriptionof the steps undertakenduringPRISMA-Pdevelopmentcan be foundin the processfollowsgeneralrecommendationsof theEQUATOR(Enhancingthe Qualityand Transparencyof healthResearch)Networkon how to developa reportingguideline,ofwhichone fundamentalpart is a internationalexpertswas heldin June 2011 in Rockville,MD, USA,to developand relatedguidancedocumentshave undergoneiterativerevisionwithinthe prisma -PGrouplistedat the end of this document;membersof the prisma -PGroupcontributedto the writingand identifyingrelevantexamplesin this final prisma -Pchecklistcontains17 numbereditems(26sub- items )that shouldbe described,at minimum,in protocolsof systematicreviewsand meta-analyses(table2 ).