Transcription of Report on the Deliberation Results - pmda.go.jp
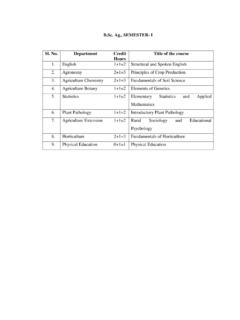
1 Report on the Deliberation Results December 3, 2014 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] Cosentyx for Subcutaneous I njection 150 mg Syringe Cosentyx for Subcutaneous I njection 150 mg [Non-proprietary name] Secukinumab (Genetical Recombination) (JAN*) [Name of applicant] Novartis Pharma [Date of application] December 26, 2013 [ Results of Deliberation ] In the meeting held on November 28, 2014, the Second Committee on New Drugs concluded that the product may be approved and that this result should be reported to the Pharmaceutical Affairs Department of the Pharmaceutical Affairs and Food Sanitation Council.
2 The re-examination period is 8 years. The drug substance and the drug product are both classified as powerful drugs, and the product is classified as a biological product. [Conditions for approval] The applicant is required to: 1. Develop a risk management plan for the product and implement it appropriately. 2. Conduct an appropriate post-marketing surveillance study to fully evaluate the long-term safety and efficacy of the product, including the occurrence of infections, etc. *Japanese Accepted Name (modified INN) This English version of the Japanese review Report is intended to be a reference material to provide convenience for users.
3 In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. The PMDA will not be responsible for any consequence resulting from the use of this English version. - Review Report November 14, 2014 Pharmaceuticals and Medical Devices Agency The Results of a regulatory review conducted by the Pharmaceuticals and Medical Devices Agency on the following pharmaceutical product submitted for registration are as follows. [Brand name] (a) Cosentyx for Subcutaneous I njection 150 mg Syringe (b) Cosentyx for Subcutaneous I njection 150 mg [Non-proprietary name] Secukinumab (Genetical Recombination) [Name of applicant] Novartis Pharma [Date of application] December 26, 2013 [Dosage form/Strength] (a) Solution for injection in a pre-filled syringe: Each syringe contains150 mg of Secukinumab (Genetical Recombination) in 1mL.
4 (b) Powder for injection in a vial for reconstitution before use:1 Each vial contains 180 mg of Secukinumab (Genetical Recombination). [Application classification] Prescription drug, (1) Drug with a new active ingredient [Chemical structure] See Figure 1 and Figure 2 below. Molecular formula: L-chain molecule: C1024H1594N280O335S6 H-chain molecule: C2268H3477N597O686S16 Molecular weight: 147, ( the protein) [Definition] Secukinumab is a recombinant human IgG1 monoclonal antibody against human interleukin-17A. Secukinumab is produced in Chinese hamster ovary cells.
5 Secukinumab is a glycoprotein (molecular weight, ca. 151,000) consisting of two molecules of H-chain ( 1-chain) containing 457 amino acid residues and two molecules of L-chain ( -chain) containing 215 amino acid residues. [Items warranting special mention] None [Reviewing office] Office of New Drug IV 1 The product is designed to ensure an extractable volume of mL of solution for injection containing 150 mg of Secukinumab (Genetical Recombination) after the lyophilized powder is reconstituted with mL of Water for Injection (Japanese Pharmacopoeia [JP]), and contains a 20% overage to compensate for loss during reconstitution.
6 Doses of the product in this Report are expressed in terms of Secukinumab (Genetical Recombination). This English version of the Japanese review Report is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. The PMDA will not be responsible for any consequence resulting from the use of this English version. Figure 1. H- and L-chains of Secukinumab (Genetical Recombination) H-chain E1: partial formation of pyroglutamic acid, H-chain N307: glycosylation, H-chain K457: partial processing L-chain C215-H-chain C230, H-chain C236-H-chain C236, and H-chain C239-H-chain C239: inter-chain disulfide bonds Solid line: intra-chain disulfide bonds L-chain H-chain 3 Figure 2.
7 Main proposed glycosylation structures Gal: Galactose, GlcNAc: N-acetylglucosamine, Man: Mannose, Fuc: Fucose 4 Review Results November 14, 2014 [Brand name] (a) Cosentyx for Subcutaneous I njection 150 mg Syringe (b) Cosentyx for Subcutaneous I njection 150 mg [Non-proprietary name] Secukinumab (Genetical Recombination) [Name of applicant] Novartis Pharma [Date of application] December 26, 2013 [ Results of review] Based on the submitted data, the efficacy of the product has been demonstrated in the treatment of psoriasis vulgaris and psoriatic arthritis in patients who have had an inadequate response to conventional therapy, and its safety is acceptable in view of its observed benefits.
8 Serious adverse drug reactions such as infections may occur following administration of the product. Therefore, prior to the use of the product, the patient s symptoms, etc. should be monitored closely and the risks and benefits of the product should be weighed. A post-marketing surveillance study, etc. to follow the patients for the occurrence of serious infections and malignancy, etc. should be conducted and the obtained information etc. should be provided accordingly to physicians and patients, etc.
9 As a result of its review, the Pharmaceuticals and Medical Devices Agency has concluded that the product may be approved for Indications and Dosage and administration as shown below, with the following conditions. [Indications] Treatment of the following diseases in patients who have had an inadequate response to conventional therapy: Psoriasis vulgaris and psoriatic arthritis [Dosage and administration] The usual adult dosage is 300 mg of Secukinumab (Genetical Recombination) by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks.
10 A dose of 150 mg may be acceptable for some patients, depending on their body weight. [Conditions for approval] The applicant is required to: 1. Develop a risk management plan for the product and implement it appropriately. 2. Conduct an appropriate post-marketing surveillance study to fully evaluate the long-term safety and efficacy of the product, including the occurrence of infections, etc. 5 Review Report (1) October 23, 2014 I. Product Submitted for Registration [Brand name] (a) Cosentyx for Subcutaneous I njection 150 mg Syringe (b) Cosentyx for Subcutaneous I njection 150 mg [Non-proprietary name] Secukinumab (Genetical Recombination) [Name of applicant] Novartis Pharma [Date of application] December 26, 2013 [Dosage form/Strength] (a) Solution for injection in a pre-filled syringe: Each syringe contains 150 mg of Secukinumab (Genetical Recombination) in 1mL.