Chapter 7 Electron Configuration And The Periodic
Found 5 free book(s)BASIC PRINCIPLES OF INORGANIC CHEMISTRY
ubblab.weebly.comChapter 2 describes the ‘Structure of the Atom’ in terms of electrons and orbitals and the build-up process to the Long Form of the Periodic Table. Chapter 3 briefly describes how the ‘Physical Properties of the El- ements’ are related to the electron configuration of the elements and
Chapter 2: Atomic Structure and Chemical Bonding
physics.uwo.caChapter 2 11 Electronegativity The electronegativity of the elements, adapted from Smith&Hashemi Electronegativity is a degree to which an atom attracts electron to itself Chapter 2 12 Chemical reactivity: valence e-s Noble gases: s2p6 configuration Ne: 1s 22s p6 Electronegative – if it is almost full, the atom has a tendency to gain
CHAPTER 1 INTRODUCTION TO ORGANIC CHEMISTRY 1.1 …
www.siue.eduIn the compound lithium fluoride, the 2s 1 electron of lithium is transferred to the 2p 5 orbital of fluorine. The lithium atom gives up an electron to form the positively charged lithium cation with 1s 2, 2s0 configuration, and the fluorine atom receives an electron to form a fluoride anion with 1s 2, 2s2, 2p 6 configuration.
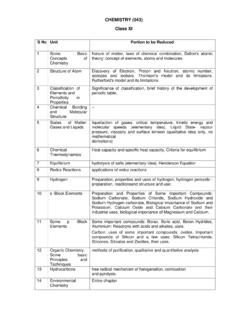
CHEMISTRY (043) Class XI
cbseacademic.nic.in2 Structure of Atom Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson's model and its limitations. Rutherford's model and its limitations 3 Classification of Elements and Periodicity in Properties Significance of classification, brief history of the development of periodic table, 4 Chemical Bonding
Coordination Chemistry III: Tanabe-Sugano Diagrams and ...
www.chem.uci.eduTo solve this problem we first need to determine the d-electron count for the [Co(NH3)6]2+ complex. Co NH 3 6 2 6NH 3 Co 2 So we have cobalt(II). Since cobalt is in the ninth column of the Periodic Table, it must be a d7complex so we can use the d7 Tanabe-Sugano diagram from the last slide. Next we need to find ∆o/B: O B