Naming Acids 1 Naming Binary Acids Naming
Found 8 free book(s)High School Chemistry Rapid Learning Series
www.rapidlearningcenter.comAcids Formulas Tutorial 08: Naming Chemical Compounds Binary Ionic Naming Polyatomic Ionic Naming Ionic with Multivalent Metals Naming Binary Covalent Naming Acids Naming Tutorial 09: Counting Molecules: The Mole The Mole Molar Mass ...
Chapter 11 Acids and Bases Practice Problems Section 11.1 ...
ion.chem.usu.eduSection 11.1 – Acids and Bases Goal: Describe and name acids and bases. Summary: An Arrhenius acid produces H+ and an Arrhenius base produces OH-in aqueous solutions. Acids taste sour, may sting, and neutralize bases. Bases taste bitter, feel slippery, and neutralize acids. Naming acids: Binary acids contain a single anion: H n X. To name:
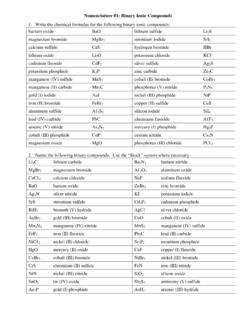
Nomenclature #1: Binary Ionic Compounds
pattersonscience.weebly.comNomenclature #3: Naming Acids 1. Name the following binary acids. These acids contain only hydrogen and one other element. Their names are always “hydro_____ic acid”. (“Hydro” tells you it is a binary acid) HI hydroiodic acid HF hydrofluoric acid H 3P hydrophosphoric acid HBr hydrobromic acid HC ℓ hydrochloric acid H
Introductory chemIstry
assets.pearsonschool.comNaming Binary Acids 146 Naming Oxyacids 147 ChEmistry in thE EnvironmEnt Acid Rain 148 5.10 Nomenclature Summary 148 Ionic Compounds 149 Molecular Compounds 149 Acids 149 5.11 Formula Mass: The Mass of a Molecule or Formula Unit 150 CHAPTER IN REvIEw 151 KEy TERMS 156 ExERCISES 156 6 ...
Naming Acids 1. NAMING BINARY ACIDS NAMING …
www.sciencegeek.netNaming Acids Acids are divided into two groups: Binary and Oxyacids. Binary acids consist of two elements. Oxyacids consist of 3 elements, one of which is oxygen. 1. NAMING BINARY ACIDS: The name of the binary acid consists of two words. The first word has three parts: the “hydro” prefix the root of the nonmetal element
CHAPTER 7 Cheical Formulas an Cheical Coons
efhshonorschemistry.weebly.com(3 × 2- = 6-). The largest common factor of the subscripts is 1. The correct formula is therefore written as follows. Al 2 O 3 Naming Binary Ionic Compounds The nomenclature, or naming system, of binary ionic compounds involves combining the names of the compound’s positive and negative ions. The
Naming Compounds - Germanna Community College
78bbm3rv7ks4b6i8j3cuklc1-wpengine.netdna-ssl.comNaming Compounds Provided by the Academic Center for Excellence 1 Updated October 2013 Naming Compounds Naming compounds is an important part of chemistry. Most compounds fall in to one of three categories- ionic compounds, molecular compounds, or acids. Part One: Naming Ionic Compounds Identifying Ionic Compounds
General Chemistry Nomenclature
www.sccollege.eduB. Binary Compounds between Two Nonmetals (molecular compounds) 1. Prefixes are used to specify the number of each atom present . i.e. 1=mono, 2=di, 3=tri, 4=tetra, 5=penta, 6=hexa, 7=hepta, 8=octa . 2. If first atom is a single atom then prefix “mono’ is omitted