Search results with tag "Manufacturing practices"
Good manufacturing practices: water for pharmaceutical use
www.who.int120 manufacturing practices for active pharmaceutical ingredients (2), and the WHO Good 121 manufacturing practices for pharmaceutical products: main principles (3). 122 123 2. Background to water requirements and uses 124 125 2.1 Water is a widely used substance in the pharmaceutical industry and other establishments 126 involved in ...
SCHEDULE M GOOD MANUFACTURING PRACTICES AND …
rajswasthya.nic.ininspection and reference and the manufacturing premises shall be used exclusively for production of drugs and/or no other manufacturing activity shall be undertaken therein except in respect of units licensed prior to 11th December’2001. PART – I Good Manufacturing Practices for Premises and Materials 1. GENERAL REQUIREMENTS --- 1.1.
Good Manufacturing Practices Questions and Answers
www.canada.caA.1 Division 2, Good Manufacturing Practices (GMP), of the Food and Drug Regulations does not specifically require manufacturing facilities for non-sterile drugs to maintain HEPA filtered air. The Regulations do require the use of equipment for adequate control over air pressure, microorganisms, dust, humidity and temperature, when appropriate.
Good Manufacturing Practices (GMP) Policy Manual
www.scbs.net.auGood Manufacturing Practices (GMP) Policy Manual Equipment Wood The use of wood in all handling and processing areas is expressly forbidden for all food contact surfaces. Glass Policy Glass No glass equipment, utensils, containers or test tubes are permitted in Coolrooms, handling areas or processing areas.
WHO GOOD MANUFACTURING PRACTICES: WATER FOR …
www.who.intworking document qas/10.379 july 2010 restricted who good manufacturing practices: water for pharmaceutical use proposal for revision draft for comments
Annex 2 WHO good manufacturing practices: water for ...
www.who.int67 Annex 2 WHO good manufacturing practices: water for pharmaceutical use1 1. Introduction 68 1.1 Scope of the document 68 1.2 Background to water requirements and uses 68
WHO GOOD MANUFACTURING PRACTICES: WATER FOR …
www.who.intworking document qas/10.379/rev.1 august 2011 restricted who good manufacturing practices: water for pharmaceutical use revised draft for comments
Good Manufacturing Practices (GMP) for Medicinal Products
cdn.intechopen.comof the pharmaceutical products. Effective implementation of GMP would also provide the cost benefit to the manufacturers, by avoiding the cost of failures such as cost of waste, of rework, of recall, of consumer compensation, of company reputation, and of regulatory action su spending operations. 3. Good Manufacturing Practices (GMP) guidelines
SSOP and GMP Practices and Programs - Sanitation …
extension.purdue.eduGood Manufacturing Practices (GMPs) contain both requirements and guidelines for manufacturing of food and drug products in a sanitary environment. The Food and Drug Administration (www.fda.gov) has developed GMPs for all foods, and that agency enforces those GMPs for all foods except meat, poultry, and egg products. The U.S. Department of
Good manufacturing practices guide for drug products
www.canada.caGood manufacturing practices guide for drug products (GUI-0001) Page 10 of 156 About quality management 4. Pharmaceutical quality system Guiding principles Do you hold an establishment licence, or run an operation governed by Part C, Division 2 of the Food and Drug Regulations? If you do, you must make sure that you comply with these
Good Manufacturing Practices Checklist
www.agriculture.pa.govCurrent Good Manufacturing Practices (GMPs) -- Food Establishment Checklist*-- * This document serves as a guide only. The official regulations can be found in 21 CFR Part 117 which can be accessible at: 1 Rev.6/2018 p.
ICH Q10 Pharmaceutical Quality System
database.ich.orgA harmonized pharmaceutical quality system applicable across ... Manufacturing Practices which are generally not repeated within the Guideline 1. Achieve product realisation 2. ... Improvements to manufacturing processes and products Training and/or realignment of …
GOOD DISTRIBUTION PRACTICES (GDP) FOR …
www.who.intprinciples of good manufacturing practice (GMP) should be applied. These include, but are not limited to, storage, distribution, transportation, packaging, labelling, documentation and record-keeping practices. The quality of pharmaceutical products can be …
Annex 3 WHO good manufacturing practices for ...
www.who.int194 2.3 In general these manufacturing facilities should be regarded as containment facilities. 2.4 The effective operation of a facility may require the combination of
Annex 3 WHO good manufacturing practices for ...
www.who.int193 1. Introduction 1.1 These guidelines set out good practices applicable to facilities handling pharmaceutical products (including active pharmaceutical
SCHEDULE M - Central Drugs Standard Control …
www.cdsco.nic.in[SCHEDULE M] [See Rules 71, 74, 76 and 78] GOOD MANUFACTURING PRACTICES AND REQUIREMENTS OF PREMISES, PLANT AND EQUIPMENT FOR PHARMACEUTICAL PRODUCTS. Note: - To achieve the objectives listed below, each licensee shall evolve appropriate
World Health Organization
www.gmpua.comWorld Health Organization Supplementary Guidelines on Good Manufacturing Practices for Heating,Ventilation and Air conditioning (HVAC) Systems for Non-sterile Dosage Forms
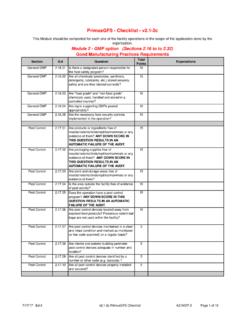
PrimusGFS - Checklist - v2.1-2c
www.primusgfs.comSection Q # Question Total Points Expectations Module 2 - GMP option (Sections 2.16 to to 2.32) Good Manufacturing Practices Requirements Equipment Cleaning 2.22.07 Is stored equipment that is …
Guidelines for Developing and (ESTRs) Ready-to-Eat (RTE ...
www.haccpalliance.orgGuidelines for Developing Good Manufacturing Practices (GMPs), Standard Operating Procedures (SOPs) and Environmental Sampling/Testing Recommendations
WHO good manufacturing practices: water for …
apps.who.int70 WHO Technical Report Series No. 970, 2012 WHO Expert Committee on Specifications for Pharmaceutical Preparations Forty-sixth report 2.4 Water sources and treated water should be monitored regularly for chemical, microbiological and, as appropriate, endotoxin contamination.
Annex 6 WHO good manufacturing practices for sterile ...
www.who.int266 is required for this. ISO 14644-2 (6) provides information on testing to demonstrate cont inued compl iance w ith the ass igned cleanl iness class ifi cation. Clean room and clean-air device monitoring
Annexure-1 GMP CHECKLIST
cdsco.nic.inSOP No.: EP-INS-004 Page 1 Annexure-1 GMP CHECKLIST (Based on WHO Good Manufacturing Practices (GMP) for active pharmaceutical ingredients stated as per
Q7 Good Manufacturing Practice Guidance for Active ...
www.fda.govThe ICH guidance Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of ...
Reflection Paper on Good Manufacturing Practice and ...
www.ema.europa.euReflection paper on Good Manufacturing Practice and Marketing Authorisation Holders . ... engaged in manufacturing and related activities (e.g. contract analysis) that are subject to EU GMP requirements. This includes holders of manufacturing and importation authorisations, as …
Q 7 Good Manufacturing Practice for Active Pharmaceutical ...
www.ema.europa.euThis document (Guide) is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the requirements
Good Manufacturing Practice (GMP) -What
www.transfusionguidelines.orgzGood Manufacturing Practice (GMP) ensures that quality is built into the organisation and processes involved in manufacture zGMP covers all aspects of “manufacture” including collection, transportation, processing, storage, quality control and delivery of the finished product
Guideline Sponsors Responsibilities IMP handling and ...
www.ema.europa.euPractice and Good Manufacturing Practice EMA/202679/2018 Page 4/6 . 21 . Introduction 22 This guideline complements the Delegated Regulation (EU) No 2017/1569 of 23 May 2017, on good 23 manufacturing practice (GMP) for investigational medicinal products (IMP) and arrangements for
Q7 Implementation Working Group ICH Q7 Guideline: Good ...
database.ich.orgICH Q7 Guideline: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Questions and Answers Current version dated 10 June 2015. Dated : 10 June 2015 Q7 Q&As i In order to facilitate the implementation of the Q7 Guidelines, the ICH Experts have developed a series of Q&As:
SUPPLIER QUALIFICATION & MANAGEMENT GUIDELINE
www.apic.cefic.orgmanufacturing practice regulations that are designed to ensure their quality, safety and efficacy. This ensures that patients worldwide and at any time can have confidence in the quality, safety and efficacy of medicines. The cGMP regulations for final medicinal products are clearly defined in each country and region.
CODEX GENERAL STANDARD FOR CONTAMINANTS AND …
www.fao.orgThe principles of Good Manufacturing Practice and Good Agricultural Practice as defined by Codex shall be used. ... 2 For the contaminants methylmercury, radionuclides, acrylonitrile and vinylchloride monomer a Codex guideline level (GL) has been established. A Codex guideline level ...
ICH HARMONISED TRIPARTITE GUIDELINE
www.ich.orgGOOD MANUFACTURING PRACTICE GUIDE FOR ACTIVE PHARMACEUTICAL INGREDIENTS ICH Harmonised Tripartite Guideline Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 10 November 2000, this guideline is recommended for adoption to the three regulatory parties to ICH
Quality Agreement for Laboratories Guideline Templates
apic.cefic.orgAPIC’s focus is on worldwide Quality, Good Manufacturing Practice (GMP) and Regulatory matters relating to APIs and intermediates. Through the years APIC has developed into a high-profile industry association with an excellent, worldwide reputation. APIC has already developed a series of guidance documents and position papers (see
Similar queries
Good manufacturing practices, Water for pharmaceutical use, Manufacturing Practices, Good, Manufacturing, Manufacturing practices: water for, Manufacturing practices: water for pharmaceutical, Requirements, Products, Pharmaceutical products, Practices and Programs - Sanitation, PHARMACEUTICAL, ICH Q10 Pharmaceutical Quality System, Manufacturing Practice, Practices, SCHEDULE M, World Health Organization, PrimusGFS - Checklist, Guidelines for Developing and ESTRs, Guidelines for Developing, Endotoxin, Class, Annexure-1 GMP CHECKLIST, Manufacturing Practice Guidance for, Guidance, Manufacturing Practice for Active Pharmaceutical, Good Manufacturing Practice, Practice, Guideline, Quality Agreement