Search results with tag "Solubility"
Hansen Solubility Parameters: Introduction and Applications
www.turi.orgSolubility Parameters (HSPs) HSPs mean we can represent each compound as a point in 3D “solubility space” Distance between HSP points in solubility space is defined as follows: With some work, it is also possible to define an interaction radius (R 0) and a reduced energy difference (RED = R a /R 0) RED > 1 Incompatible, RED < 1
Techniques to improve the solubility of poorly soluble drugs
www.ijplsjournal.compoorly water soluble drugs, which can subsequently affect the in vivo absorption of drug. Currently only 8% of new drug candidates have both high solubility and permeability. Because of solubility problem of many drugs the bioavailability of them gets affected and hence solubility enhancement becomes necessary.
TWO-BASE EXTRACTION OF BENZOIC ACID, 2-NAPHTHOL, …
www.sas.upenn.eduSolubility 0.08g/100mL H2O 1 benzoic acid pKa=4.17 Solubility 0.34g/100mL H2O 5 N-(4-nitrophenyl)-benzamide Solubility 0.08g/100mL H2O NH O N+ O-O O O NH 3 N+ O-O 2 benzoate ion 4 4-nitroanilinium ion Unknown Sample 1,3,5 Dissovle unknown sample in ether. Extract with aqueous hydrochloric acid. aqueous phase 4, water, HCl (aq) 1,5,ether Basifyt ...
Identifying an Unknown Compound by Solubility, Functional ...
www1.udel.eduSolubility tests can suggest the size and polarity of an unknown compound and the presence of basic or acidic functional groups. A compound’s solubility in aqueous acid or base involves ionization of the compound and, therefore, a chemical …
EXPERIMENT 2- QUALITATIVE ANALYSIS OF AMINO …
chem.boun.edu.tr1) Solubility Tests: The solubility of amino acids and proteins is largely dependent on the solution pH. The structural changes in an amino acid or protein that take place at different pH values alter the relative solubility of the molecule. In acidic solutions, both amino and carboxylic groups are protonated.
Sample Exercise 13.1 Predicting Solubility Patterns
www.centrallyon.orgPlan With the information given, we can use Henry’s law, Equation 13.4, to calculate the solubility, S CO 2. Solve S CO 2 = kP CO 2 = (3.4 10 2 mol/L-atm)(4.0 atm) = 0.14 mol/L = 0.14 M Check The units are correct for solubility, and the answer has two significant figures consistent with both the partial pressure of CO
Table Solubility - University of Texas at Austin
www.chemprep.cm.utexas.eduThis is the solubility table that is available for use within ALEKS. Feel free to have this table with you when you take the assessment or when you are in learning mode in ALEKS. Even so, you will save a lot of time (now and later in UT Chem classes) if you commit these two solubility rules to memory: • Compounds with Group 1 cations and NH 4
Triton® X Surfactants - search.cosmobio.co.jp
search.cosmobio.co.jpSolubility in nonpolar organic solvents, such as aliphatic hydrocarbons, is generally the reverse of solubility in water. Most of the products are soluble in aromatic hydrocarbons and in polar organic solvents, such as alcohols, ether, etc. The below table shows the qualitative solubility behavior of these materials.
Chapter 9: Phase Diagrams
web.eng.fiu.edusolubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9 ...
SAFETY DATA SHEET
content.interlinebrands.comWater solubility Flash point 100% Revision Date: 07-Apr-2015 Solubility in other solvents ... pH value Autoignition temperature Evaporation rate No information available . Decomposition temperature No information available No information available . Viscosity of Product < 10 cps 10.4 - 11.2 ... Inhalation No known effect. Skin contact No known ...
SILICONE FLUIDS - Gelest
www.gelest.comCompatibility Properties Water solubility insoluble Hydrocarbon aromatic/ soluble/ solubility aliphatic partial Optical Properties Refractive Index n D 25 range 1.393-1.403 Release & Surface Tension, range 19.2-21.6 Wettability Properties dynes/cm Wear/Lubricity Properties Four ball wear, mm at 75°C, 40 kg. load 2-3 steel on steel, one hr.
Changing pH in Soil - University of California, Davis
vric.ucdavis.eduBetween pH 6.0 and 6.5, most plant nutrients are in their most available state. A nutrient must be soluble and remain soluble long enough to successfully travel through the soil solution into the roots. Nitrogen, for example, has its greatest solubility between soil pH 4 and soil pH 8. Above or below that range, its solubility is seriously ...
Acid-Base Extraction - University of Massachusetts Amherst
people.chem.umass.edusalt of carboxylic acid - low solubility in ether - high solubility in water In the experiment done in this lab, a mixture of a carboxylic acid (stronger acid), a phenol (weaker acid), and a neutral compound will be separated by acid-base extractions. The separated compounds
WRITING CHEMICAL EQUATIONS
chymist.comThe rules governing the solubility of common salts are given below: THE SOLUBILITY RULES 1. All sodium, potassium, and ammonium salts are soluble in water. 2. The nitrates, chlorates, and acetates of all metals are soluble in water. Silver acetate is sparingly soluble. 3. The chlorides, bromides, and iodides of all metals except lead, silver ...
Determination of Water- and Fat-Soluble Vitamins by HPLC
assets.thermofisher.comSolubility and stability of water- and fat-soluble vitamins The physical properties of water- and fat-soluble vitamins, such as solubility and stability in different solvents, are summarized in Table 1. Knowledge of these properties is important for sample preparation and analysis. Riboflavin (vitamin B 2) is easily dissolved in a basic
Studies on solubility of curcumin
www.ijplsjournal.comCurcumin is practically water insoluble and have poor bioavailability. In order to improve its water solubility, solid dispersions of curcumin were prepared by both hot melt method and solvent evaporation method. In hot melt method, PEG-4000, PEG-6000 were chosen as carriers. The ratios of drug to carrier were 1:1, 1:4 and 1:8.
Why purify proteins? - UAB
www.uab.eduSolubility of Proteins • Solubility of a protein depends on ionic strength of the solution • Ionic strength is defined as I = (1/2) ∑ i C i Z i 2 • The sum is over all ionic species • Because the charge Zi is squared, divalent and trivalent ions contribute greatly to I. • Ci is the molar concentration of species i
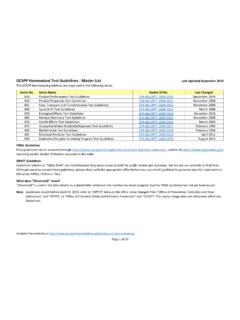
OCSPP Harmonized Test Guidelines ‐ Master List Last ...
www.epa.gov830.7840 Water Solubility: Column Elution Method; Shake Flask Method 796.1840 63‐8 105 712‐C‐96‐041 March 1998 830.7860 Water Solubility, Generator Column Method 796.1860 63‐8 none 712‐C‐96‐042 March 1998 830.7950 Vapor Pressure 796.1950 63‐9 …
PRACTICAL LAB MANUAL
jru.edu.inSolubility: soluble or insoluble in different solvents like-water, HCl, NaOH, NaHCO3. The solubility of a compound in different reagents (s olvents) gives important information about its category/class. Take 0.1 g or 2-3 drops of the given sample in about 3 mL of a solvent in a test
The Solubility of Nitrogen and Air in Liquids - NIST
srd.nist.govThe Solubility of Nitrogen 'and Air in Liquids Rubin Battino, Timothy R. Rettich,a) and Toshihiro Tominagab) , Department of Chemistry, Wright State University, Dayton, Ohio 45435
Experiment # 10: Solubility Product Determination
www.ulm.eduIn order for a more precise determination of Ksp, the solution mixtures are designed such that the various Q values are rather close together. Caution: lead salts are toxic, so be careful handling the solutions, and make sure to wash your hands after you complete the experimental work. Preparation of lead iodide mixtures
Laboratory Risk Assessment Tool - Stanford University
ehs.stanford.eduFeb 06, 2021 · ☐ Exothermic, with potential for fire, (gases, excessive heat, or runaway reaction ☐ ☐Endothermic, with potential for freezing solvents decreased solubility or heterogeneous mixtures ☐ ☐ ☐Gases produced ☐ ☐Hazardous reaction intermediates/products ☐ Hazardous side reactions . Hazardous Processes ☐ Generation of air contaminants
Pharmacokinetics: The Absorption, Distribution, and X ...
downloads.lww.comsorbed. The dissolving of a tablet or capsule is referred to as dissolution. Manufacturing processes and the water solubility of the drug affect dissolution rates. Highly water-soluble medications dissolve more readily in the gastrointestinal (GI) tract, while fat-soluble drugs dissolve more slowly.
PROPOSAL TO WAIVE IN VIVO BIOEQUIVALENCE …
www.who.intThe API is not prone to precipitation after its dissolution due to its good solubility under all pH conditions likely to be found in the upper gastrointestinal tract. The high permeability assures the complete uptake (> 90%) of the API during its passage through the small intestine. The fast dissolution of the product
CHEMISTRY Module 1 Fundamentals of Chemistry
sites.ntc.doe.gova. Mixture c. Solubility e. Solution b. Solvent d. Solute f. Equilibrium 2.7 STATE Le Chatelier’s principle. 2.8 DEFINE the following terms: a. ppm c. Density b. Molarity d. Normality 2.9 BALANCE chemical equations that combine elements and/or compounds. CH-01 Page viii Rev. 0
Physical and Chemical Agents for Microbial Control
www.mmc.gov.bd– solubility in water or alcohol, stable – broad spectrum, low toxicity – penetrating – noncorrosive and nonstaining – affordable and readily available 12/30/13 Dr. Shyamal Kr Paul, Streilization. Levels of Chemical Decontamination • High-level germicides – kill endospores; may be
Cambridge International Examinations Cambridge ...
papers.gceguide.com3 samples of dilute hydrochloric acid with different volumes of aqueous sodium hydroxide. In each case, the student measures the change in temperature to test if the reaction is ... 16 At 400 °C the reaction between hydrogen and iodine reaches an equilibrium. The reaction is exothermic. H 2(g) + I ... compound formula solubility in water ...
2015 TX STAAR Grade 5 Science Released Book
tea.texas.govSolubility can be used to separate objects that dissolve in water from objects that do not. J. Physical state can be used to separate objects that are solids from objects that are not. Page 11: GO ON 9: An African savanna is a grassland with shrubs and a few small trees. It has warm
CHEMISTRY 12 SOLUBILITY REVIEW QUESTIONS
msbunney.weebly.com11. Barium nitrate reacts with potassium sulphate solution and forms insoluble barium sulphate. What volume of 0.40 M Ba(NO 3) 2 solution is required to precipitate the sulphate ions in 25.00 mL of 0.80 M K 2 SO 4? 12. A 225 mL sample of tap water containing the chloride ion requires 36.42 mL of 0.100 M AgNO 3 to titrate.
Ion chromatography analysis methods and issues
www.waters.comDepending on the sample matrix, other ions influence solubility. K sp = [A-][C+] pK sp = -log K sp [AC] Example: Analysis of Cl in a sample matrix containing Ag, or Analysis of SO 4 in a sample with high Ca. K sp for AgCl is 9.75 implying that Cl is not in solution K sp for CaSO 4 is 5.04 implying that only a portion of the SO
Hydrogen Sulfide in Aqueous Solvents - NIST
srdata.nist.govThe solubility of hydrogen sulfide in water has been investigated by numerous workers since the nineteenth century. Some of this early work (1-3) is in good agreement with more recent work. Hydrogen sulfide exists, in aqueous solution, in equilibrium with its ions. Solubilities which have been compiled correspond to bulk solubilities. For this
SAFETY DATA SHEET Polypropylene Pellets/ Resin
pinnaclepolymers.comPolypropylene Resin/Pellets Date of Issue: July 2015 Revision: 0 Vapor pressure No data available Vapor density No data available Relative density No data available Solubility Insoluble in water Partition coefficient: n‐octanol/water Insoluble in water and octanol Auto ignition temperature > 340 C
1 Buffer Preparation - MD Anderson Cancer Center
www.mdanderson.orgNote: pH adjusted by NaOH is essential for solubility. Autoclavable. 3. TAE DNA Electrophoresis Buffer (50 X) (2 M Tris, 50 mM EDTA) 4 L 968 g Tris 228.4 ml glacial acetic acid 400 ml 0.5 M EDTA 8.0 To make 1x TAE 20 L, add 400 ml 50X buffer into 19.6 L ddH2O. 4. SDS-PAGE Gel Solutions Vol (L) Tris (g) HCl (ml) 10% SDS (ml)
Chemistry formula and data book
www.qcaa.qld.edu.auPhysical constants and unit conversions Absolute zero Atomic mass unit Avogadro’s constant Ideal gas constant Ionic product constant for water (at 298 K) Molar volume of an ideal gas (at STP) Specific heat capacity of water (at 298 K) ... Solubility of selected compounds at 298 K
AP Chemistry 2013 Scoring Guidelines
secure-media.collegeboard.orgAnswer the following questions about the solubility of some fluoride salts of alkaline earth metals. (a) A student prepares 100. mL of a saturated solution of MgF 2 by adding 0.50 g of solid MgF 2 to 100. mL of distilled water at 25°C and stirring until no more solid dissolves. (Assume that the volume of the undissolved MgF 2 is negligibly small.)
Chapter 1 Organic Compounds: Alkanes
www.angelo.eduNormal physical state Gases, liquids, or low melting-point solids Usually high melting-point solids Flammability Often flammable Usually nonflammable Solubility in water Often low Often high Conductivity of aqueous solutions Nonconductor Conductor Table 1.1 Properties of typical organic and inorganic compounds.
Reflection paper on the dissolution specification for ...
www.ema.europa.eulimited by solubility / intrinsic dissolution of the active substance, i.e. BCS II and IV, suitable quality attributes may be particle size of the active substance or other attr ibutes that would have an impact on the . in vivo. dissolution. For a finished product where the . in vivo.
NIT-7 YSTEMATIC QUALITATIVE NALYSIS - NCERT
ncert.nic.in(iii) Determination of cations by reactions carried out in solution (wet tests) and confirmatory tests. Preliminary examination of a salt often furnishes important information, which ... Solubility of a salt in water and the pH of aqueous solutions give important information about the nature of ions present in the salt. If a solution of the salt is
Content and Format of Chemistry, Manufacturing, and ...
www2.rsna.orgApr 14, 2010 · • (e.g., solubility, partition coefficient, ionic charge, dissociation constants, etc.) – Evidence to support proposed chemical structure, including stereochemistry. ... • Some product attributes may not be critical to the safety or efficacy of …
Form and Stability of Aluminum Hydroxide Complexes in ...
pubs.usgs.govAluminum has a particularly strong tendency to hydrolize in solution, and at any pH above 3.5 various combinations of aluminum with hydroxide ions occur. The purpose of this investigation was to determine the effect of pH on aluminum solubility and …
IS 3025-39 (1991): Methods of sampling and test (physical ...
law.resource.orgCD the basis of their common solubility in trichlorotrifluoroethane. The methods covered in this standard are suitable for .assessment ofbiological Iiquids, mineral hydrocarbons, industrial wastewater ortreated effluents containing these materials. The methods shall Dot be applicable to low boiling fractions that volatalize at temperatures ...
Solubility of Hydrocarbons in Physical Solvents - BR&E
www.bre.comsolubility data very closely and the be used to investigate factors affecting hydrocarbon solubility in physical solvents. Ethylene Glycol GPA RR-117 [7], RR-149 [8], RR-137 [9] contain equilibrium data for solubility of methane, propane, n-heptane, ABSTRACT This paper compares the solubility of hydrocarbons in several physical solvents such
Solubility: Importance, Measurements and Applications
www.crystallizationsystems.comThe TV method is the most suitable method for determining the temperature dependent solubility line of a compound in a solvent. Upon heating a suspension of known composition, the temperature at which all crystals are dissolved marks a point on the solubility line. After a recrystallization step through cooling, the measurement can be repeated.
Solubility Product Constant
repository.uobabylon.edu.iqS is an example of the common ion effect. In general, any ionic equilibrium is affected by a substance producing an ion involved in the equilibrium. Calculating the solubility of a Slightly Soluble Salt in a Solution of a Common Ion (a) Calculate the molar solubility of barium fluoride, BaF 2, in water at 25 oC. The K sp at 25 oC is 1.0 x 10-6 ...
Solubility chart KEY - Mrs. Horne's Science Site
sciencewithhorne.weebly.comReading a Solubility Chart 1) The curve shows the # of grams of solute in a saturated solution containing 100 rnL or 100 g of water at a certain temperature. 2) Any amount of solute below the line indicates the solution is unsaturated at a certain temperature o 3) Any amount of solute above the line in which all of the solute has dissolved
Solubility table - sciencenet.cn
image.sciencenet.cnB Ammonium hydrogen phosphate (NH4)2HPO4 42.9 62.9 68.9 75.1 81.8 89.2 97.2 106 110 112 121 Ammonium hydrogen sulfate NH4HSO4 100 Ammonium hydrogen tartrate NH4HC4H4O6 1.88 2.7 Ammonium iodate NH4IO3 2.6 14.5 Ammonium iodide NH4I 155 163 172 182 191 200 209 219 229 250 Ammonium nitrate NH4NO3 118 150 192 242 297 344 421 499 580 740 871 …
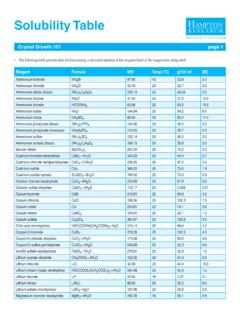
Solubility Table - Hampton Research
hamptonresearch.comPotassium fluoride KF 58.10 20 34.9 6.0 Potassium formate HCOOK 84.12 20 117.7 14.0 Potassium iodide KI 166.00 25 103.2 6.2 Potassium nitrate KNO3 101.10 25 30.3 3.0 Potassium phosphate dibasic K2HPO4 174.18 22 52.3 4.0 Potassium phosphate monobasic KH2PO4 136.09 22 …
Similar queries
Solubility, Solubility of poorly soluble, Poorly water soluble, Of solubility, Solubility enhancement, EXPERIMENT 2- QUALITATIVE ANALYSIS OF AMINO, Table Solubility, The solubility, Solubility rules, Triton® X Surfactants, Solvents, Hydrocarbons, Temperature, Effect, FLUIDS, Acid-base, Salt, Rules, THE SOLUBILITY RULES, Determination, Water, Water solubility, Method, Why purify proteins, S olvents, Experiment # 10: Solubility Product Determination, Experimental, Gases, Absorption, Dissolution, Drugs, Aqueous, Iodine, Sulphate solution, Sulphate, Solution, Ion chromatography analysis methods and issues, CaSO 4, Hydrogen Sulfide in Aqueous Solvents, Resin, Buffer Preparation, MD Anderson Cancer Center, Constants, Product, Solids, Aluminum Hydroxide, Aluminum, Aluminum solubility, Physical, Solubility of Hydrocarbons in Physical Solvents, Solubility Product, Ammonium, Solubility Table