Search results with tag "Electrochemistry"
Chemistry Notes for class 12 Chapter 3 Electrochemistry
ncerthelp.comElectrochemistry Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry 1. Production of metals like Na, Mg. Ca and Al. 2.
Ch. 13 Fundamentals of Electrochemistry - Yonsei University
chem.yonsei.ac.kr13.1 13:20 Anal. Chem. by Prof. Myeong Hee Moon Ch. 13 Fundamentals of Electrochemistry 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : …
Basic Concepts in Electrochemistry
www.engr.uconn.edu5 What is electrochemistry? Electrochemistry is defined as the branch of chemistry that examines the phenomena resulting from combined chemical and electrical effects.
UNIT II - ELECTROCHEMISTRY & CORROSION - GRIET
www.griet.ac.in1 UNIT II - ELECTROCHEMISTRY & CORROSION Electrochemistry is a branch of chemistry which deals with interconversion of electrical energy to chemical energy and vice versa.
Impact Factor for 2016 of Electrochemistry Journals
www.ise-online.orgImpact Factor for 2016 of Electrochemistry Journals The official (ISI Web of Knowledge/Journal Citation Reports) Impact Factor (IF) of Electrochimica Acta for 2016 has been published: 4.798.The journal achieved 84,704 total cites over 2,341 citeable items ranking it at 4th place in the Electrochemistry subject area. Other measures of …
the electochemistry of corrosion cut
www.npl.co.uk1 [ELECTROCHEMISTRY OF CORROSION/BM] THE ELECTROCHEMISTRY OF CORROSION Edited by Gareth Hinds from the original work of J G N Thomas INTRODUCTION The surfaces of all metals (except for gold) in air are covered with oxide films.
Experiment 9 Electrochemistry I – Galvanic Cell
chemistry.uccs.edu9-1 Experiment 9 Electrochemistry I – Galvanic Cell Introduction: Chemical reactions involving the transfer of electrons from one reactant to another are called oxidation-reduction reactions or redox reactions.In a redox reaction, two half-reactions occur; one reactant gives up electrons (undergoes oxidation) and another reactant gains electrons (undergoes reduction).
General Chemistry II Jasperse Electrochemistry. Extra ...
web.mnstate.eduElectrochemistry. Extra Practice Problems Oxidation Numbers p1 Free Energy and Equilibrium p10 Balancing Redox; Electrons Transferred; Oxidizing Agents; Reducing Agents p2 K Values and Voltage p11 Spontaneous Voltaic Electrochemical Cells p4 Nonstandard Concentrations and Cell Potential p11 Cell Potentials p5 Electrolysis p12
Experiment 9 Electrochemistry I – Galvanic Cell
www.uccs.edu9-1 Experiment 9 Electrochemistry I – Galvanic Cell Introduction: Chemical reactions involving the transfer of electrons from one reactant to another are called oxidation-reduction reactions or redox reactions.In a redox reaction, two half-reactions occur; one reactant gives up electrons (undergoes oxidation) and another reactant gains electrons (undergoes reduction).
Battery Range Summary - EnerSys
www.enersys-emea.comConstruction • Electrochemistry optimised for high rate discharge applications • High performance positive plate grids designed to resist corrosion, prolong active life and for efficient recharge • Negative plates provide perfect balance with the positive plates to ensure optimum
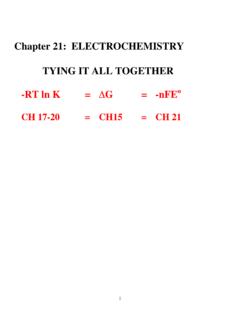
Chapter 21: ELECTROCHEMISTRY TYING IT ALL TOGETHER
laude.cm.utexas.edu"The stronger the oxidizing agent the more easily a species is reduced" "The stronger the reducing agent, the more easily a species is oxidized." ... This latter fact is subject to change without warning depending upon the type of book you read, so be cautious. But remember
ElectrElectrochemistrochemistryy ...
ncert.nic.in65 Electrochemistry As mentioned earlier (Class XI, Unit 8) a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous
A Guide to Lithium-Ion Battery Safety - IEEE Web Hosting
cmte.ieee.orgSafety characteristics vary by Li-ion electrochemistry Overcharged (delithiated) positive can become unstable Passivation layer (SEI) can break down above 100°C 7 A Guide to Lithium-Ion Battery Safety - Battcon 2014 Battcon 2008 – “Understanding Lithium -Ion Technology”
Accu-Chek Advantage: Electrochemistry for Diabetes …
currentseparations.comAccu-Chek Advantage illustrates many fundamental References By carefully optimizing a design based on fundamental Because of this, the instrument is a medical management
Jerzy Haber Institute of Catalysis and Surface Chemistry ...
issis2018.krakow.plJerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences Foundation “Pro-Kataliza” under the auspices of the International Society of Electrochemistry
ELECTROCHEMISTRY CALENDAR
www.ise-online.orgLast update: 01 September 2018 1 ELECTROCHEMISTRY CALENDAR Please send information on Meetings directly to the Special Issues Editor of Electrochimica Acta (Sergio Trasatti) by
Electrochemistry
www.chem1.com1.1 Electroneutrality Nature seems to strongly discourage any process that would lead to an excess of positive or negative charge in matter. Suppose, for example, that we immerse a piece of zinc metal in pure water. A small number of zinc atoms go into solution as Zn ions, leaving their electrons behind in the metal: Zn(s) → Zn 2+ + 2 e–
Electrochemistry of Semiconductors - Current Separations
currentseparations.comThe major interest in semicon-ductor electrodes is due to the pho-toelectrochemical properties of the semiconductor electrolyte interface; that is, the generation of currents
Electrochemistry - NCERT
www.ncert.nic.inIn Class XI, Unit 8, we had studied the construction and functioning of Daniell cell (Fig. 3.1). This cell converts the chemical energy liberated during the redox reaction Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) (3.1) to electrical energy and has an electrical potential equal to 1.1 V when concentration of Zn2+ and Cu2+ ions is unity (1 mol dm–3)*.
Similar queries
Electrochemistry, Electrochemistry Electrochemistry, Of Electrochemistry, Ch. 13 Fundamentals of Electrochemistry, Concepts, UNIT II - ELECTROCHEMISTRY & CORROSION, Factor for 2016 of Electrochemistry Journals, Of corrosion, ELECTROCHEMISTRY OF CORROSION, Galvanic Cell, Battery Range Summary, Corrosion, Stronger, Warning, Unit, Accu, Chek Advantage: Electrochemistry for Diabetes, Chek, Catalysis and Surface Chemistry, Polish Academy of Sciences, ELECTROCHEMISTRY CALENDAR, Acta, Electrochemistry of Semiconductors