Search results with tag "Galvanic cell"
CHEMISTRY (862) - CISCE
cisce.org(i) Electrochemical cells: introduction, redox reactions (principle of oxidation and reduction in a cell). (ii) Galvanic cells - introduction; representation, principle oxidation – reduction. Mechanism of production of electric curren t in a galvanic cell. (iii) Measurement of potential. Single electrode potentials.
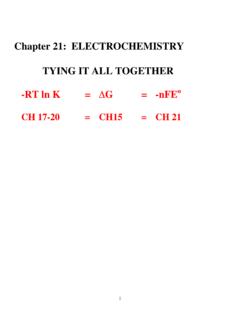
Chapter 21: ELECTROCHEMISTRY TYING IT ALL TOGETHER
laude.cm.utexas.edu6. Galvanic Cell and Electrolytic Cels. There are two kinds of electrochemical cells, those that occur spontaneously and those that require work to occur. This is simply the consequence of having reactions that are: spontaneous ( ∆ G < 0 is a battery or a galvanic cell) and reactions that are non spontaneous ( ∆ G> 0 is an electrolytic cell)
Experiment 9 Electrochemistry I – Galvanic Cell
chemistry.uccs.eduA galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and produces an electric current. In order for the transfer of electrons in a redox reaction to produce an electric current and be useful, the electrons are
ElectrElectrochemistrochemistryy ...
ncert.nic.inSuch a device is called a galvanic or a voltaic cell. If an external opposite potential is applied in the galvanic cell [Fig. 3.2(a)] and increased slowly, we find that the reaction continues to take place till the opposing voltage reaches the value 1.1 V [Fig. 3.2(b)] when, the reaction stops altogether and no current flows through the cell.
Electrochemistry
www.ncert.nic.inSuch a device is called a galvanic or a voltaic cell. If an external opposite potential is applied in the galvanic cell [Fig. 3.2(a)] and increased slowly, we find that the reaction continues to take place till the opposing voltage reaches the value 1.1 V [Fig. 3.2(b)] when, the reaction stops altogether and no current flows through the cell.
White Paper: Material Selection and Compatibility ...
www.emerson.com1.0 Introduction The potential for damage due to corrosion is an important concern in the design of instrumentation for ... form of corrosion is galvanic corrosion. A combination of a cathode, an anode, and an electrolyte must ... This combination of cathode, anode, and electrolyte is called a galvanic cell. Simply stated, a galvanic cell ...
Faraday’s Law 1 Experiment 8: Copper Electroplating and ...
www.colby.eduIn a galvanic cell (e.g. a battery) the cathode is positively charged. In an electrolytic cell, the external current source forces the cathode to be negative. Figure 2: Copper electroplating cell. The object to be plated is placed at the cathode. The anode is a strip of copper. The electrolyte is 1.0 M copper sulfate in 1.0 M sulfuric acid.
Experiment 9 Electrochemistry I – Galvanic Cell
www.uccs.edu9-1 Experiment 9 Electrochemistry I – Galvanic Cell Introduction: Chemical reactions involving the transfer of electrons from one reactant to another are called oxidation-reduction reactions or redox reactions.In a redox reaction, two half-reactions occur; one reactant gives up electrons (undergoes oxidation) and another reactant gains electrons (undergoes reduction).
Ion-Selective Electrode Determination of Fluoride Ion
www.csun.eduI. Introduction A. Ion-Selective Electrodes The amount of a specific ion contained in an aqueous solution can be determined by direct potentiometric measurement of the voltage of a galvanic cell such as shown below. reference electrode analyte solution ion selective electrode
Galvanic Cell Notation Example - UMKC
g.web.umkc.edu1 Galvanic Cell Notation • Half-cell notation – Different phases are separated by vertical lines – Species in the same phase are separated by commas • Types of electrodes ¾Active electrodes – involved in the electrode half-reaction (most metal electrodes)