Search results with tag "Implantable"
Dental Equipment and Implantable Pacemakers and …
www.bostonscientific.comFeb 02, 2009 · Active implantable medical devices—Electromagnetic compatibility—EMC test protocols for implantable cardiac pacemakers and implantable cardioverter defibrillators, pp 76-80. 4Brand, HS, Entjes, ML, Nieuw Amerongen, AV, van der Hoeff, EV, Schrama, TAM. Interference of electrical dental equipment with Implantable Cardioverter-defibrillators.
CARDIAC PACEMAKERS IMPLANTABLE CARDIOVERTER …
www.cardiovascular.abbottInsertion of a single transvenous electrode, permanent pacemaker or implantable defibrillator J1 5222 $7,641 33217 Insertion of 2 transvenous electrodes, permanent pacemaker or implantable defibrillator J1 5222 $7,641 33215 Repositioning of previously implanted transvenous pacemaker or implantable defibrillator
Incorrect Billing of HCPCS L8679 - Implantable ...
www.cms.govJan 29, 2020 · Electro-acupuncture devices and implantable neurostimulators are two separate devices, and coding electro-acupuncture devices as implantable neurostimulators is incorrect. Also, HCPCS L8679, when appropriately billed for an implantable neurostimulator pulse generator, should be submitted with the appropriate
Fiche pratique Chambre à Cathéter Implantable (CCI)
www.cpias-auvergnerhonealpes.frChambre à Cathéter Implantable (CCI) Schéma d’une CCI. 2 2015 Pose de l’aiguille de Huber ... de 30 mois se référer au guide des bonnes pratiques de l’antisepsie chez l’enfant (SF2H - 2007). ... Chambre à Cathéter Implantable (CCI) Fiche pratique-3 2015 -
WALL CONNECTOR, NEMA 14-50 INSTALLATION MANUAL
www.tesla.commedical or implantable electronic devices, such as an implantable cardiac pacemaker or an implantable cardioverter defibrillator. Check with your electronic device manufacturer concerning the effects that charging may have on such electronic devices before using the Wall Connector. Cautions Caution: Do not use private power generators as a ...
CARDIAC PACEMAKERS IMPLANTABLE CARDIOVERTER …
www.cardiovascular.abbott33218 Repair of single transvenous electrode, permanent pacemaker or implantable defibrillator 5.82 $400 NA 33220 Repair of 2 transvenous electrodes for permanent pacemaker or implantable defibrillator 5.90 $386 NA 33234 Removal of transvenous pacemaker electrode(s); single lead system, atrial or ventricular 7.66 $500 NA
Online Appendix Common Procedures and Associated ...
jaccjacc.acc.orgImplant or generator replacement, CIED (cardiac implantable electronic device) ☒ ☐ Implant, subcutaneous ICD (implantable cardioverter defibrillator) ☒ ☐ ☐ ☐ Ablation, SVT (supraventricular tachycardia) ☒ ☐ ☐ ☐ Implant, ILR (implantable loop recorder) ☒ ☐ ☐ ☐
MDCG 2019-14
ec.europa.euimplantable ophthalmologic devices) or the physical or technological principle of the device (e.g. MDA 0302 Active non-implantable devices utilising non-ionizing radiation or MDA 0315 Software). Therefore, there are cases where more than one specific code might apply to a device (e.g. surgical laser for refractive surgery of the eye).
CARDIAC DEVICE MONITORING - Medtronic
europe.medtronic.comimplantable defibrillator system (Do not report 93284 in conjunction with 93287, 93289) 93295 Interrogation device evaluation(s) (remote), up to 90 days; single, dual, or multiple lead implantable defibrillator system with interim analysis, review(s) and report(s) by a physician or other qualified health care professional
Hearing Aids and Devices Including Wearable, Bone …
www.uhcprovider.comWhat is being requested (e.g., bone anchored, semi-implantable, implantable, etc.) The type of hearing loss (sensorineural vs. conductive or mixed) Severity and frequencies affected Whether or not member is a candidate for an air -conduction hearing aid For replacement of any components, indicate date of initial purchase and the reason for
Exercise Instructions: Pacemakers & Implantable ...
www.med.umich.eduImplantable Cardioverter Defibrillators (ICDs) Always follow the advice of your provider (doctor/nurse practitioner/physician assistant). The instructions below are general and should only be done with their approval. General Guidelines Target heart rate (HR) will not exceed 150 beats per min (bpm) for most patients.
NCD 20.4 Implantable Cardiac Defibrillators (ICDs)
www.cms.govMar 03, 2020 · qualify for an Automatic Implantable Cardioverter Defibrillator (AICD) but they also do not have to have “active heart failure” in order to append one of these codes. Patients with a cardiomyopathy and a reduced ejection fraction, who experience an episode of active or acute
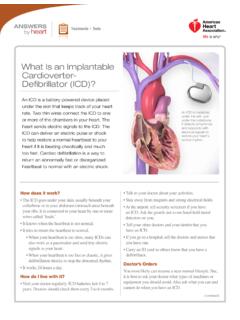
What Is an Implantable Cardioverter- Defibrillator (ICD)?
www.heart.orgWhat Is an Implantable Cardioverter- Defibrillator (ICD)? ANSWERS by heart Treatments + Tests An ICD is implanted under the skin, just under the collarbone. It detects arrhythmias and responds with electrical signals to restore your heart's normal rhythm.
Medical device patient information leaflets and implant cards
www.tga.gov.auimplantable medical device. means a medical device (other than an active implantable medical device) that is intended by the manufactu rer: (a) to be, by surgical intervention, wholly introduced into the body of a human being, and to remain in place after the procedure; or
NSW Guidelines for Deactivation of Implantable
www.aci.health.nsw.gov.au5 ACI NSW Guidelines for the Deactivation of Implantable Cardioverter Defibrillators at the End of Life 4. Aim These guidelines aim to assist clinicians to manage ICD deactivation, and facilitate a peaceful death, for patients at the end of life.
ADVANCED IMAGING - AIM Specialty Health
aimspecialtyhealth.comevidence-based criteria for medical necessity determinations where possible. In the process, multiple ... osseous assessment of the calvarium, skull base, and maxillofacial bones, including detection of calvarial and facial bone structures; and imaging of midline ... implantable devices such as pacemakers or defibrillators, a potential need for ...
Guidance on applying human factors and usability ... - GOV.UK
assets.publishing.service.gov.ukevidence may come to light while a device is being used in clinical practice that the design ... assessment and mitigation of potential patient and user safety risks; also in the ... 2002, Annex 1 (for active implantable medical devices) [as modified by Schedule 2A to the UK MDR 2002] lay down the essential requirements of medical devices, to ...
発作性房室ブロックの 臨床的特徴
new.jhrs.or.jp(implantable loop recorder : ILR)の使用頻度が増加しているため,発作性房室ブロックの自然発作が 心電図に記録されるようになってきた.ILRで確認される発作性房室ブロックは,長時 …
CPR and External Defibrillation for Pacemaker /or ...
www.bostonscientific.comJun 30, 2008 · Yes. Although implantable pacemakers and defibrillators are designed to withstand external defibrillation, the implanted device can sustain damage if the external defibrillation electrode pads are placed too close to or directly over the device. Use the lowest energy output of external defibrillation equipment that is clinically acceptable. SUMMARY
新しい「失神の診断・治療ガイドライン(2012 年改訂版)」 …
square.umin.ac.jp植え込み型ループレコーダー(implantable loop recorder:ILR)がわが国でも普及し,その有用 性が認識されている。また,長年の懸案であっ たチルト試験(head-up tilt test)が保険適応と なり,日本循環器学会の「失神の診断・治療の
標準的神経治療:めまい - jsnt.gr.jp
www.jsnt.gr.jpb. 植え込み型ホルター心電図(implantable loop recorder : ILR) 3.神経調節性失神と心原性失神の鑑別 1) 迷走神経緊張が影響する疾患 a. 冠攣縮性狭心症 b. Brugada症候群 c. 心房細動 d. 迷走神経誘発性心室頻拍 2) 交感神経緊張が影響する疾患 a. QT延長症候群(LQTS ...
Cardiac Event Monitoring - UHCprovider.com
www.uhcprovider.comImplantable Loop Recorder : Devci e used to detect abnormal heart rhythms. It is placed under the skin and continuously records the heart’s electrical activity. The recorder can transmit data to the physician’s offci e to help with monitoring. An
Going Home After Getting a Pacemaker or Defibrillator
www.uhn.ca• an implantable loop recorder (ILR) • a biventricular defibrillator (CRT-D) • a pulse generator replacement (PGR or battery replacement) • a lead repositioned • a new lead • a pocket revision • an explanted pacemaker • a laser lead extraction Form: D-5100. 2
NYHA Class II or III Heart Failure: Who Will Need an ...
file.scirp.orgImplantable Cardioverter Defibrillator (ICD) ICD is a battery-operated device, which is placed in a pouch under the skin of chest, abdomen or collar bone and has a battery unit, which generates the pulse and 1 or 2 lead(s) placed in right ventricle …
CMS Manual System
www.cms.gov12399.1 NCD 20.4 Implantable Cardiac Defibrillators (ICDs) Contractors shall add Place of Service (POS) 24, Ambulatory Surgical Centers (ASCs), to current edits for the NCD effective February 15, 2018.
Cardiovascular INSIDE THIS GUIDE
www.bostonscientific.comfor “Implantable Devices Charged to Patients”, available for cost reporting periods since May 1, 2009. As CMS uses data from this cost center to establish OPPS payments, it is important for providers to document device costs in this cost center …
Peri-Procedure Management of Anticoagulants Page 1 of 29
www.mdanderson.orgImplantable cardioverter-defibrillator/pacemaker lead extraction Left atrial appendage occlusion device Pericardiocentesis Diagnostic coronary angiography via femoral access Electrophysiology testing and/or ablation Pacemaker or defibrillator placement Right heart catheterization Supraventricular tachycardia ablation
INSTRUCTIONS FOR USE Streamer Pro 1.3AStreamer Pro 1
www.oticon.comInterference and implantable devices The Streamer is designed to comply with the most stringent Standards of International Electromagnetic Compatibility. However, the Streamer might cause interference with ... loop breaks, it cannot be repaired and must be replaced immediately. Consult your Hearing Care Professional for a replacement. 17 Neck loop
Standards for continuous cardiac monitoring in hospital ...
bhrs.comStandards for continuous cardiac monitoring in ‐ ... VTs in this setting can be slower than implantable cardioverter defibrillator (ICD) detection zones or nominal telemetry alarm thresholds. ... A system for either printing or uploading ECG data to …
NSW Guidelines for Deactivation of Implantable
aci.health.nsw.gov.audevices also have the potential to provide a biventricular pacing mode (also known as cardiac resynchronisation therapy), which helps to coordinate the left and right ventricular activity aiming to improve cardiac function and symptoms in patients with ventricular dysynchrony. As the indications for ICD implantation expand the
Billing and Coding Guidelines for Magnetic Resonance ...
downloads.cms.govICD-9 code V45.02 (automatic implantable cardiac defibrillator) or ICD-9 code V45.01 (cardiac pacemaker), NOTE: Effective for claims with dates of service on and after October 1, 2013, providers report the
Cardiac Device Monitoring Services
www.cardiovascular.abbottPacemaker, Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy (CRT) • Reported no more than once every 90 days. A period is established by the initiation of the remote monitoring or the 91st day of monitoring and extends for the subsequent 90 days for which remote monitoring is occurring
CMS Manual System
www.cms.govcondition, the item is a prosthetic device & billed to the DME REGIONAL Carrier. ... E0350 - E0352 Electronic Bowel Irrigation System DME REGIONAL Carrier E0370 Heel Pad DME REGIONAL Carrier E0371 - E0373 Decubitus Care Equipment DME REGIONAL Carrier ... E0616 Implantable Cardiac Event Local Carrier Recorder 3.
Bonnes pratiques d’une chambre a catheter implantable
oncocentre.orgSurveillance de la CCI lors de perfusions prolongées Changement d’aiguille tous les 8 jours Pansement occlusif et stérile en cas de perfusion continue avec visualisation du point de ponction (à refaire s’il n’est plus occlusif) Lignes de perfusion : respect du système clos en limitant au maximum connexions et robinets, ligne de tubulure complète
IV INJECTION GUIDELINES FOR CT CONTRAST
www.med.umich.eduBard-PowerFlow- implantable apheresis IV Port . a. Power injection (preferred method) b. Hand injection : Powe Picc and Power Ports can be hand injected by . a. CT Tech and b. RN, MD, PA . Please make sure it states Power injectable. Please make sure it states Power injectable . a. Up to 5 cc/sec . b. Test flush for ease of
Cardiac Pacemaker Evaluation Services (NCD 20.8.1 ...
www.uhcprovider.comProgramming device evaluation (in person) with iterative adjustment of the implantable device to test the function of the device and select optimal permanent programmed values with analysis, review and report by a physician or other qualified health care professional; single lead pacemaker system or leadless pacemaker system in one cardiac chamber
Prosthetic Devices, Wigs, Specialized, Microprocessor or ...
www.uhcprovider.comImplantable devices/prostheses, such as artificial heart valves, are not prosthetics. If covered, these devices would be covered as a surgical service. Prosthetic Devices An initial or replacement prosthetic device is a covered health care service when all of the following criteria are met:
Amiodarone: Guidelines for Use and Monitoring - American ...
www.aafp.orgDec 01, 2003 · [Evidence level A, meta-analysis] The benefit ... implantable cardioverter-defibrillators (ICDs) ... or cough should elicit a prompt assessment for pulmonary toxicity. Congestive heart fail-
MANAGEMENT OF HEART FAILURE with Reduced Ejection …
www.derbyshiremedicinesmanagement.nhs.ukICD implantable cardiovascular defibrillators LFT liver function test ... • a clinical assessment of functional capacity, fluid status, cardiac rhythm, and cognitive and ... (up-titrate to maximum tolerated evidence-based dose) These above treatments may be combined if indicated
MAGNET OPERATION - Medtronic
wwwp.medtronic.comcardiac device systems and who consult with the patients’ cardiologists. OVERVIEW The Model 9466 Patient Magnet is a blue coated, ring‐shaped magnet. All Medtronic Implantable Pulse Generator (IPG) and Cardiac Resynchronization Pacemakers (CRT‐P) Models,
Cardiac Pacemakers: Single Chamber and Dual Chamber ...
www.uhcprovider.comQuestions and Answers ..... 3 References ... Chapter 12; § 30.4 B. Electronic Analyses of Implantable Cardioverter-defibrillators and Pacemakers . CMS Transmittal(s) Transmittal 187, Change Request 9078, Dated 12/10/2015 (National Coverage Determination (NCD) for Single Chamber and ...
MEDICAL DEVICES INDUSTRY
www.miti.gov.myD High Risk Heart Valve, Implantable Defibrillator - The Medical Devices Regulations 2012 is aimed at protecting patients and other customers from substandard and unregistered medical devices. Medical devices are categorised into A, B, C and D …
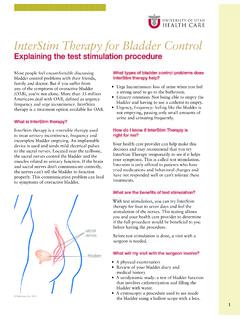
InterStim Therapy for Bladder Control - University of Utah
healthcare.utah.eduincomplete bladder emptying. An implantable device is used and sends mild electrical pulses to the sacral nerves. Located near the tailbone, the sacral nerves control the bladder and the muscles related to urinary function. If the brain and sacral nerves don’t communicate correctly, the nerves can’t tell the bladder to function properly.
Fact Sheets Index - CDHO
cdho.orgImplantable Cardioverter Defibrillators Infective Endocarditis Influenza J Joint Replacement Juvenile Arthritis Juvenile Rheumatoid Arthritis (Juvenile Arthritis) K Kidney Disease (CKD) Kidney Failure (CKD) Kissing Disease (Mononucleosis) L Leukemia Lichen Planus Liver Disease Lung Cancer Lupus
Implantable Cardioverter Defibrillator (ICD) and Cardiac ...
www.cardiovascular.abbottImplantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy for Defibrillators (CRT-D) Procedures . FREQUENTLY USED CPT‡ CODES - HOSPITAL OUTPATIENT AND PHYSICIAN SERVICES . HOSPITAL NAME …
Implantable Automatic Defibrillators (NCD 20.4)
www.uhcprovider.comOther For patients that are candidates for heart transplantation on the United Network for Organ Sharing (UNOS) transplant list awaiting a donor heart, coverage of ICDs, as with cardiac resynchronization therapy, as a bridge-to-transplant to prolong
Similar queries
Implantable, For implantable, Chambre, Bonnes pratiques, Implantable Cardiac, Device, Implantable Defibrillator, Cardiac implantable electronic device, CARDIAC DEVICE MONITORING, Devices Including Wearable, Bone, Implantable Cardioverter Defibrillators, Defibrillator, Guidelines for Deactivation of Implantable, Guidelines, Deactivation of Implantable, Deactivation, Evidence, Assessment, Defibrillators, Implantable loop recorder, And External Defibrillation for Pacemaker /or, Implantable loop, Cardiac Event Monitoring, Recorder, Implantable Cardioverter, INSIDE THIS GUIDE, Management, INSTRUCTIONS FOR USE, Loop, Cardiac, Biventricular, Implantable Cardioverter Defibrillator, CMS Manual System, Electronic, Bonnes pratiques d’une chambre a catheter implantable, Cardiac Pacemaker Evaluation Services NCD, Implantable device, Prosthetic Devices, Wigs, Specialized, Microprocessor, HEART FAILURE with Reduced Ejection, Cardiac device, Questions, Answers, InterStim, University of Utah, Fact Sheets Index, Implantable Automatic Defibrillators NCD, Cardiac resynchronization therapy