Transcription of Unit - NCERT
1 After studying this Unit, you will beable to name alcohols, phenols andethers according to the IUPAC system of nomenclature; discuss the reactions involved inthe preparation of alcohols fromalkenes, aldehydes, ketones andcarboxylic acids; discuss the reactions involved inthe preparation of phenols fromhaloarenes, benzene sulphonicacids, diazonium salts andcumene; discuss the reactions forpreparation of ethers from(i) alcohols and (ii) alkyl halidesand sodium alkoxides/aryloxides; correlate physical properties ofalcohols, phenols and ethers withtheir structures; discuss chemical reactions of thethree classes of compounds onthe basis of their , phenols and ethers are the basic compounds for theformation of detergents, antiseptics and fragrances, ,,,,, PhenolsPhenolsPhenolsPhenolsPhenolsandan dandandand EEEEE thertherthertherthersssssAlcoholsAlcohol sAlcoholsAlcoholsAlcohols,,,,, PhenolsPhenolsPhenolsPhenolsPhenolsandan dandandand EEEEE thertherthertherthersssssYou have learnt that substitution of one or morehydrogen atom(s)
2 From a hydrocarbon by another atomor a group of atoms result in the formation of an entirelynew compound having altogether different propertiesand applications. Alcohols and phenols are formedwhen a hydrogen atom in a hydrocarbon, aliphatic andaromatic respectively, is replaced by OH group. Theseclasses of compounds find wide applications in industryas well as in day-to-day life. For instance, have youever noticed that ordinary spirit used for polishingwooden furniture is chiefly a compound containinghydroxyl group, ethanol. The sugar we eat, the cottonused for fabrics, the paper we use for writing, are allmade up of compounds containing OH groups. Justthink of life without paper; no note-books, books, news-papers, currency notes, cheques, certificates, etc.
3 Themagazines carrying beautiful photographs andinteresting stories would disappear from our life. Itwould have been really a different alcohol contains one or more hydroxyl (OH)group(s) directly attached to carbon atom(s), of analiphatic system (CH3OH) while a phenol contains OHgroup(s) directly attached to carbon atom(s) of anaromatic system (C6H5OH).The substitution of a hydrogen atom in ahydrocarbon by an alkoxy or aryloxy group(R O/Ar O) yields another class of compounds knownas ethers , for example, CH3 OCH3 (dimethyl ether). Youmay also visualise ethers as compounds formed by2022-23324 Chemistrysubstituting the hydrogen atom of hydroxyl group of an alcohol orphenol by an alkyl or aryl this unit, we shall discuss the chemistry of three classes ofcompounds, namely alcohols, phenols and alcohols may be further classified according to thehybridisation of the carbon atom to which the hydroxyl group isattached.
4 (i)Compounds containing 3 COH spbond: In this class of alcohols,the OH group is attached to an sp3 hybridised carbon atom of analkyl group. They are further classified as follows:Primary, secondary and tertiary alcohols: In these three types ofalcohols, the OH group is attached to primary, secondary andtertiary carbon atom, respectively as depicted below:Allylic alcohols: In these alcohols, the OH group is attached toa sp3 hybridised carbon adjacent to the carbon-carbon doublebond, that is to an allylic carbon. For exampleBenzylic alcohols: In these alcohols, the OH group is attachedto a sp3 hybridised carbon atom next to an aromatic ring. classification of compounds makes their study systematic andhence simpler.
5 Therefore, let us first learn how are alcohols, phenolsand ethers classified?Alcohols and phenols may be classified as mono , di , tri- orpolyhydric compounds depending on whether they contain one, two,three or many hydroxyl groups respectively in their structures asgiven Mono, Di,Tri orPolyhydricalcoholsMonohydricDihydricTr ihydric2022-23325 Alcohols, Phenols and EthersAllylic and benzylic alcohols may be primary, secondary or tertiary.(ii) Compounds containing 2 COH sp bond: These alcohols contain OH group bonded to a carbon-carbon double bond, , to avinylic carbon or to an aryl carbon. These alcohols are also knownas vinylic alcohol: CH2 = CH OH2CH3CH3(i)H C2 CHCH OH2(ii)CH3CH2 CHOH2(iii)CHOHCH3(iv)CH2 OHCHCH3(v)CHOHCHCCH3CH3(vi) the following as primary, secondary and tertiary allylic alcohols in the above QuestionsIntext QuestionsIntext QuestionsIntext QuestionsIntext Nomenclature(a) Alcohols.
6 The common name of an alcohol is derived from thecommon name of the alkyl group and adding the word alcohol to example, CH3OH is methyl Mono, DiandtrihydricphenolsEthers are classified as simple or symmetrical, if the alkyl or arylgroups attached to the oxygen atom are the same, and mixed orunsymmetrical, if the two groups are different. Diethyl ether,C2H5OC2H5, is a symmetrical ether whereas C2H5 OCH3 and C2H5OC6H5are unsymmetrical to IUPAC system (Unit 12, Class XI), the name of an alcoholis derived from the name of the alkane from which the alcohol is derived,by substituting e of alkane with the suffix ol . The position ofsubstituents are indicated by numerals.
7 For this, the longest carbonchain (parent chain) is numbered starting at the end nearest to thehydroxyl group. The positions of the OH group and other substituentsare indicated by using the numbers of carbon atoms to which these areattached. For naming polyhydric alcohols, the e of alkane is retainedand the ending ol is added. The number of OH groups is indicated byadding the multiplicative prefix, di, tri, etc., before ol . The positions of OH groups are indicated by appropriate locants, , HO CH2 CH2 OHis named as ethane 1, 2-diol. Table gives common and IUPAC names of a few alcohols as : Common and IUPAC Names of Some AlcoholsCH3 OHMethyl alcoholMethanolCH3 CH2 CH2 OHn-Propyl alcoholPropan-1-olIsopropyl alcoholPropan-2-olCH3 CH2 CH2 CH2 OHn-Butyl alcoholButan-1-olsec-Butyl alcoholButan-2-olIsobutyl alcohol2-Methylpropan-1-oltert-Butyl alcohol2-Methylpropan-2-olHO H2C CH2 OHEthylene glycolEthane-1,2-diolGlycerolPropane -1, 2, 3-triolCompoundCommon nameIUPAC nameCyclic alcohols are named using the prefix cyclo and consideringthe OH group attached to C (b) Phenols.
8 The simplest hydroxy derivative of benzene is is its common name and also an accepted IUPAC name. As structureof phenol involves a benzene ring, in its substituted compounds theterms ortho (1,2- disubstituted), meta (1,3-disubstituted) and para(1,4-disubstituted) are often used in the common , Phenols and EthersCommon namePhenolo-Cresolm-Cresolp-CresolIUPAC namePhenol2-Methylphenol3-Methylphenol4- MethylphenolDihydroxy derivatives of benzene are known as 1, 2-, 1, 3- and1, nameCatecholBenzene-diol1,2-ResorcinolBe nzene-diol1,3-Hydroquinone or quinolBenzene-diol1,4-IUPAC name(c) Ethers: Common names of ethers are derived from the names of alkyl/aryl groups written as separate words in alphabetical order and adding theword ether at the end.
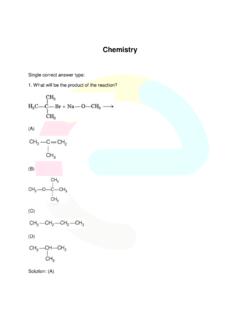
9 For example, CH3OC2H5 is ethylmethyl : Common and IUPAC Names of Some EthersCompoundCommon nameIUPAC nameCH3 OCH3 Dimethyl etherMethoxymethaneC2H5OC2H5 Diethyl etherEthoxyethaneCH3 OCH2CH2CH3 Methyl n-propyl ether1-MethoxypropaneC6H5 OCH3 Methyl phenyl etherMethoxybenzene(Anisole)(Anisole)C6H 5 OCH2CH3 Ethyl phenyl etherEthoxybenzene(Phenetole)C6H5O(CH2)6 CH3 Heptyl phenyl ether1-PhenoxyheptaneCH3 CHO3 CHCH3 Methyl isopropyl ether2-MethoxypropanePhenyl isopentyl ether3- MethylbutoxybenzeneCH3 O CH2 CH2 OCH3 1,2-Dimethoxyethane 2-Ethoxy--1,1-dimethylcyclohexane2022-23 328 ChemistryIf both the alkyl groups are the same, the prefix di is added before the alkylgroup.
10 For example, C2H5OC2H5 is diethyl to IUPAC system of nomenclature, ethers are regarded ashydrocarbon derivatives in which a hydrogen atom is replaced by an OR or OAr group, where R and Ar represent alkyl and aryl groups,respectively. The larger (R) group is chosen as the parent names of a few ethers are given as examples in Table (i) 4-Chloro-2,3-dimethylpentan-1-ol(ii)2-Et hoxypropane(iii)2,6-Dimethylphenol(iv)1- Ethoxy-2-nitrocyclohexaneNO2OC H25 Example (i)(iii)(ii)CH3 CHO CH2CH3CH3(ii)CH3 CHCH OH2 ClCH CHCH3CH3(i)(iv) Name the following compounds according to IUPAC QuestionIntext QuestionIntext QuestionIntext QuestionIntext Question(i)(ii)(iii)(iv)(v)In alcohols, the oxygen of the OH group is attached to carbon by asigma ( ) bond formed by the overlap of a sp3 hybridised orbital ofcarbon with a sp3 hybridised orbital of oxygen.