CHAPTER 12: COORDINATION CHEMISTRY IV: REACTIONS …
Rate = (k1 + k2[As(C6H5)3])[Co(NO)(CO)3] This reaction shows both first and second order kinetics; the ligand substitution likely occurs via two mechanisms. The first order reaction appears to be a dissociative reaction or a solvent-assisted dissociation of CO, followed by a fast addition of As(C6H5)3. The other path shows first
Download CHAPTER 12: COORDINATION CHEMISTRY IV: REACTIONS …
Information
Domain:
Source:
Link to this page:
Please notify us if you found a problem with this document:
Documents from same domain
Standard practices for Fmoc-based solid-phase …
www.chem.uci.eduStandard practices for Fmoc-based solid-phase peptide synthesis in the Nowick laboratory (Version 1.6.1) Adam G. Kreutzer and Patrick J. Salveson
Phases, Based, Practices, Standards, Synthesis, Solid, Peptides, Fmoc, Standard practices for fmoc based, Standard practices for fmoc based solid phase peptide synthesis
An Introduction to Fluorescence Spectroscopy
www.chem.uci.eduAn Introduction to Fluorescence Spectroscopy 3 Table of Contents ... molecular extinction coefficient of the compound; in other words, the corrected
Molecular, Spectroscopy, Fluorescence, Fluorescence spectroscopy
CHAPTER 10: COORDINATION CHEMISTRY II: BONDING
www.chem.uci.edu10.8 Co(II) is d 7. In tetrahedral complexes, it is generally high spin and has 3 unpaired electrons; in In tetrahedral complexes, it is generally high spin and has 3 unpaired electrons; in octahedral complexes, it is also typically high spin and also has 3 unpaired electrons; in square
Dynamic Light Scattering Training
www.chem.uci.eduwww.atascientific.com.au Mie Theory › Mie theory is an exact description of how spherical particles of all sizes and optical properties scatter light › When particles become larger than /10, the scattering changes from being isotropic to a distortion in the
Introduction to Kinetics and Equilibrium
www.chem.uci.eduIntroduction to Kinetics and Equilibrium Kinetics and equilibrium are two of the most ... At iitill tii lPClA system initially containing only PCl 5 at t ti f 0 100t a concentration of 0.100 M has a Q c = 0, which is less than 0.030. What are the equilibrium concentrations
Introductionto FourierTransform InfraredSpectrometry ...
www.chem.uci.eduof material present. With modern software algorithms, infrared is an excellent tool for quantitative analysis. Older Technology. The original infrared instruments were of the . dispersive. type. These instruments separated the individual frequencies of energy emitted from the infrared source. This was accomplished by the use of a prism or grating.
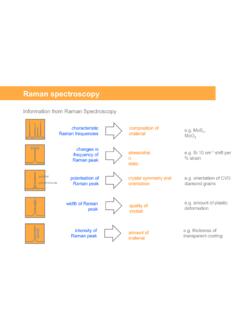
Raman spectroscopy
www.chem.uci.eduFrom the Renishaw Raman software the user can select: • a fixed grating measurement with a spectrum 'window' of 400 cm-1 to 1000 cm (configuration dependent) • a unique 'extended scanning' facility allowing the user to choose any Raman shift range up to about 10000 cm-1 (configuration dependent). Essential for extended range
A Second Application of Symmetry
www.chem.uci.eduWe can use character tables to determine the symmetry of all 18 motions and then assign them to translation, rotation, or vibration. We can also tell which vibrations are IR or Raman active. Procedure: 1. Assign x,y,z coordinates to each atom. 2. Determine how each axis transforms for every class of symmetry operation in the group.
Coordination Chemistry II: Ligand Field Theory Continued
www.chem.uci.eduCoordination Chemistry II: Ligand Field Theory Continued Chapter 10 Wednesday, November 25, 2015. Adding Metal Electrons e g e g * a1g a1g * t1u t1u * t2g Metal ions typically have some valence electrons that can be accommodated in the metal d …
Zinc-EDTA Titration
www.chem.uci.eduZinc-EDTA Titration You would like to perform a titration of 50.00 mL of a 1.00 x 10-4 M Zn2+ solution with a 1.00 x 10-4 M EDTA solution. What is pZn at the equivalence point? Log K f for the ZnY2-complex is 16.5. Both solutions are buffered to a pH of 10.0 using
Related documents
Repeatable Battery for the Assessment of Neuropsychological …
images.pearsonclinical.comFigure Recall 16.1 (2.9) 13.5 (3.3) 13.5 (3.3) 13.6 (4.0) 12.5 (4.2) 11.4 (4.1) General Issues in Interpreting Performance on the RBANS Certain index scores can be significantly affected by relatively minor changes on certain subtests.This is particularly
BTEC FIRST HEALTH AND SOCIAL CARE - Pearson …
qualifications.pearson.com2 Key features of the BTEC First suite of qualifications 5 3 Pearson BTEC Level 1/Level 2 First Certificate, Extended Certificate and Diploma in Health and Social Care 11 Rationale for the Pearson BTEC Level 1/Level 2 First Certificate, Extended Certificate and Diploma in Health and Social Care 11 4 Qualification structures 17
First, Health, Social, Care, Pearson, Btec, Btec first health and social care, 5 3 pearson
SEVENTH EDITION Using Multivariate Statistics - Pearson
www.pearsonhighered.comPEARSON and ALWAYS LEARNING are exclusive trademarks owned by Pearson Education, Inc. or its affiliates, in the U.S., and/or other countries. ... 3.2.5.3 Unequal n and Nonorthogonality 42 3.2.5.4 Fixed and Random Effects 43 3.2.6 Specific …
SPECIFICATION - Edexcel
qualifications.pearson.comThe Pearson BTEC Level 1/Level 2 Tech Award in Digital Information Technology (Qualification Number: 603/2740/6), is for learners who want to acquire technical knowledge and technical skills through vocational contexts by studying the knowledge, understanding and skills
Physics 100A Homework 4 – Chapter 5 Newton’s First Law A) …
www.csun.eduPhysics 100A Homework 4 – Chapter 5. Newton’s First Law . A)If a car is moving to the left with constant velocity then the net force applied to the car is zero. B) An object cannot remain at rest unless the net force acting on it is zero. C) An object has constant acceleration if the net force acting on it is constant. Understanding Newton’s Laws . A)An object cannot remain at rest ...