Search results with tag "Equilibria"
Chapter 8, Acid-base equilibria - Boston University
www.bu.eduChapter 8, Acid-base equilibria Road map of acid-base equilibria On first encounter, the study of acid-base equilibria is a little like a strange land with seemingly
Chapter 16: Acid-Base Equilibria
www2.onu.eduChapter 16: Acid-Base Equilibria In the 1st half of this chapter we will focus on the equilibria that exist in aqueous solutions containing: weak …
Properties of Gases and Liquids
www.eng.uc.edu8-9 Multicomponent Vapor-Liquid Equilibria at Low Pressure / 8.32 8-10 Determination of Activity Coefficients / 8.42 8-11 Phase Equilibrium with Henry’s Law / 8.111 8-12 Vapor-Liquid Equilibria with Equations of State / 8.120 8-13 Solubilities of Solids in High-Pressure Gases / 8.158 8-14 Liquid-Liquid Equilibria / 8.159 8-15 Phase ...
Chapter 17: Overview of the Chapter Solubility & Complex ...
www2.onu.eduChapter 17: Solubility & Complex Ion Equilibria The goal of this chapter is to understand the equilibria that exist between ionic solids and their ions in solution, and factors that affect that equilibrium.
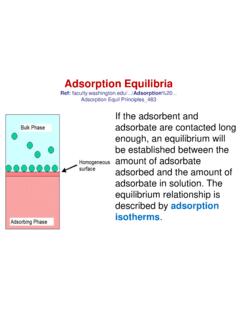
Adsorption Equilibria - Marmara Üniversitesi
mimoza.marmara.edu.trAdsorption Equilibria The adsorption capacity of activated carbon may be determined by the use of an adsorption isotherm which can take multiple forms. Isotherms are found by doing lab tests.
Carbonate equilibria in natural waters - Chem1
www.chem1.comCarbonate equilibria in natural waters A Chem1 Reference Text Stephen K. Lower Simon Fraser University Contents 1 Carbon dioxide in the atmosphere 3 2 The carbonate system in aqueous solution 3
Game Theory Lecture Notes
personal.psu.eduFinding Nash Equilibria in Simple Games78 8. A Note on Nash Equilibria in General80 Chapter 6. An Introduction to Optimization and the Karush-Kuhn-Tucker Conditions83 iii. iv CONTENTS 1. A General Maximization Formulation84 2. Some Geometry for Optimization86 …
Calcium and phosphate compatibility: • The influence of ...
ahfs.ashp.orgequilibria relevant to calcium and phosphate compat-ibility. The keys to understanding the chemical reactions and relative risks for calcium phosphate precipitation are as follows: • The clinically relevant dissocia-tion equilibria for which the pK a2 of phosphoric acid is 7.2 (i.e., the pH at which the concentrations or, ther-
Experiment 1 Chemical Equilibria and Le Châtelier’s Principle
www.colby.eduExperiment 1 Chemical Equilibria and Le Châtelier’s Principle A local theatre company is interested in preparing solutions that look like blood for their upcoming
Advanced GCE Unit F325: Equilibria, Energetics and Elements
www.ocr.org.ukOxford Cambridge and RSA Examinations Mark Scheme for January 2013 GCE Chemistry A Advanced GCE Unit F325: Equilibria, Energetics and Elements
Get help and support AS AND A-LEVEL CHEMISTRY - AQA
filestore.aqa.org.ukThe AS exams are similar in style to the A-level exams, testing a subset of the same content, with less difficulty. ... • Exampro: a searchable bank of past AQA exam questions ... 3.1.6 Chemical equilibria, Le Chatelier’s principle and K c page 22
Acid-base Equilibria and Calculations - Chem1
www.chem1.com• Acid and base strengths. 1.2 Acid and base strengths. The equilibrium constants that define the strengths of an acid and of a base are K. a = [H. 3. O +][OH ]
iess B3 H-O - Columbia University
www.columbia.edu(T); player 1 wins a dollar from player 2 if their choices are the same, and loses a dollar to player 2 if they are not. This game has no pure-strategy Nash equilibria.
CARBONATE EQUILIBRIA - UC Davis
lawr.ucdavis.eduSoil Chemistry 5-3 Section 5- Carbonate Chemistry -3 + 2- 3 o - 10.3 - HCO 3 ( )H CO ( ) = K = 10 ( HCO ) (4) As for every aqueous reaction the acid base relationship between the …
Hyperquad simulation and speciation (HySS): a utility ...
www.hyperquad.co.ukCoordination Chemistry Reviews 184 (1999) 311–318 Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially
ADSORPTION AND ION EXCHANGE S preferential …
www.pacontrol.com15.2. ION EXCHANGE EQUILIBRIA 497 TABLE 15.2-(continued) (b) Typical Properties of Union Carbide Type X Molecular Sieves Ncniiiiinl piirv hilk I Irill ld IGpiililiriiiiii
CHAPTER 13 GAME THEORY AND COMPETITIVE …
www.uh.eduChapter 13: Game Theory and Competitive Equilibrium 187 Firm 1 chooses Low, neither will have an incentive to change (900 > 50 for Firm 1 and 600 > -30 for Firm 2). Both outcomes are Nash equilibria.
Download Free Lecture Notes-Pdf Link-I - NPTEL
nptel.ac.inChapter 7 Vapour-Liquid Equilibria . 7.1 Introduction . Both the general criterion of thermodynamic equilibrium as well as the specific condition of equality
ENZYMES
www.enzymes.at3.6 Subunits and Quaternary Structure / 65 3.7 Cofactors in Enzymes / 68 3.8 Summary / 71 References and Further Reading / 74 4 Protein‒Ligand Binding Equilibria 76
DESIGN OF ABSORBER - DAV University
www.davuniversity.organd physical absorption is governed by the physical equilibria. Chapter 5 ABSORBER 59 5.2.2 Chemical Absorption In this type of absorption as soon as a particular component comes in contact with the absorbing liquid a chemical reaction take place. ... LIQUID HOLD UP: Because of the liquid on each plate there may be a Urge quantity of the liquid ...
Wednesday 13 June 2012 – Morning
www.ocr.org.ukWednesday 13 June 2012 – Morning A2 GCE CHEMISTRY A F325 Equilibria, Energetics and Elements INSTRUCTIONS TO CANDIDATES † The insert will be found in the centre of this document.
OCR A Level H432/01 Chemistry A June 2018
revisionscience.com6 OCR 2018 11 The table below shows standard entropies, Sө. Substance CO(g) H 2(g) CH 3OH(l) Sө / J mol –1 K –1 197.6 130.6 239.7 What is the entropy change, ∆Sө, in J mol–1 K –1, for the following reaction? CO(g) + 2H 2(g) CH 3OH(l) A –219.1 B –88.5 C +88.5 D +219.1 Your answer [1] 12 The redox equilibria for a hydrogen–oxygen fuel cell in alkaline solution are …
Chapter 9: POLYPROTIC ACID-BASE EQUILIBRIA
webhost.bridgew.edu3/28/2014 5 pH Calculations : Diprotic Acids and Bases Problem: Find the pH and concentrations of H 2SO 3, HSO 3-and SO 3 2-in each of the following solutions:(a) 0.050 M H 2SO 3 (b) 0.050 M NaHSO 3, and (c) 0.050 M Na 2SO 3 Note that for diprotic acids and bases , there are 3 species in
EXPERIMENT # 4 SHIFT HAPPENS OR INVESTIGATING …
www.slcc-science.org1 EXPERIMENT # 4 SHIFT HAPPENS... OR INVESTIGATING CHEMICAL EQUILIBRIA Objective To investigate the effect of stress on a chemical system at equilibrium and explain
1400-Fm 9/9/99 7:37 AM Page i CModern Analytical ...
gtu.geAcid–Base Equilibria 219 7G.4 Liquid–Liquid Extractions Involving Metal Chelators 221 7H Separation versus Preconcentration 223 7I Key Terms 224 ... 6G.4 pH of a Monoprotic Weak Acid 160 6G.5 pH of a Polyprotic Acid or Base 163 6G.6 Effect of Complexation on Solubility 165 6H Buffer Solutions 167 6H.1 Systematic Solution to Buffer
Property Package Descriptions
www.razifar.comenhanced to improve the vapour-liquid-liquid equilibria calculations for water-hydrocarbon systems, particularly in dilute regions. The model is an improvement over previous attempts which were limited in the region of validity. The modification is based …
pH in Drinking-water - WHO
www.who.intwaters, is controlled by the carbon dioxide–bicarbonate–carbonate equilibrium system. An increased carbon dioxide concentration will therefore lower pH, whereas a decrease will cause it to rise. Temperature will also affect the equilibria and the pH. In pure water, a decrease in pH of about 0.45 occurs as the temperature is raised by 25 °C.
Big-Picture Introductory Conceptual Questions
web.mnstate.eduDisrupted Equilibria p12 Solving for an Equilibrium Concentration Given K and Other Equilibrium Concentrations p7 Answers p15 Big-Picture Introductory Conceptual Questions ... The equilibrium constant for the formation of calcium carbonate from the ions in …
Introduction to Alloy Phase Diagrams
www.asminternational.orgcibility in both the liquid and solid states The Gibbs phase rule applies to all states of matter (solid, liquid, and gaseous), but when the effect of pressure is constant, the rule reduces to: f=c-p+ 1 The stable equilibria for binary systems are sum- …
I. Acidic and Basic water solutions: Dissociation of water O
www.sas.upenn.eduVI. Acid-Base Equilibria I. Equilibrium constant for the dissociation of a weak acid, K a Remember, weak acids only slightly dissociate: HB ⇔ H+ (aq) + B-(aq) The products are a proton and a conjugate base. Since the reactants are in equilibrium with the products, we can write an equilibrium expression: K a = [H +][B-] ----- [HB] K
CHAPTER 3: PHASE EQUILIBRIA
classes.engineering.wustl.edu3.2 Vapor-Liquid Equilibrium The ratio of the composition measure such as (mole fraction) in the vapor phase to that in the liquid phase at equilibrium is referred to as the K-value. Note that Ky is dimensionless. i eq i yi x y K ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ = (1) where yi is the mole fraction of species i in the vapor phase and xi is the liquid.
Chapter 8, Acid-base equilibria
www.bu.eduThis equation is called the autoionization of water and its equilibrium constant is known as the water autoionization constant Kw. At 25°C it is equal to Kw =@H3O+D@OH-D=1.0μ10-14 Now, we have seen that the equilibrium constant of a sum of two reactions is the product of the equilibrium constants of the summed reactions.
Part 1: Seawater carbonate chemistry
www.pmel.noaa.govAbout 90% of this is present as bicarbonate ion, the proportion of carbonate ion is about a factor of 10 less (~10%), and that of unionised carbon dioxide yet another factor of 10 less (<1%). As a result of the equilibria between these various species (see below), seawater is buffered (weakly) with respect to changes in
21.7 HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES
www.saplinglearning.com1008 CHAPTER 21 • THE CHEMISTRY OF CARBOXYLIC ACID DERIVATIVES less stable than their corresponding hydroxy acids. Consequently, the lactonization equilibria for these compounds favor instead the hydroxy acids.
Similar queries
Chapter 8, Acid-base equilibria, Boston University, Chapter 16: Acid-Base Equilibria, CHAPTER, Solutions, Liquid equilibria, Liquid, Overview of the Chapter Solubility, Solubility & Complex Ion Equilibria, Equilibria, Adsorption Equilibria, CARBONATE EQUILIBRIA, Carbonate, Nash equilibria, Calcium, Calcium phosphate, Experiment 1 Chemical Equilibria and Le Châtelier, Unit F325: Equilibria, Energetics and Elements, Style, Exam questions, Acid-base Equilibria and Calculations, ACID, Columbia University, Aqueous, Simulation, Investigation of equilibria involving soluble, ADSORPTION, ION EXCHANGE S preferential, GAME THEORY AND COMPETITIVE, Equilibrium, NPTEL, Chapter 7 Vapour, Enzymes, Chemistry, Chapter 9: POLYPROTIC ACID-BASE EQUILIBRIA, SHIFT HAPPENS OR INVESTIGATING, SHIFT HAPPENS... OR INVESTIGATING CHEMICAL EQUILIBRIA, Effect, Base Equilibria, Polyprotic Acid, Base, Property Package Descriptions, Acidic and Basic water solutions, HYDROLYSIS OF CARBOXYLIC ACID DERIVATIVES