Search results with tag "Colby college"
Some Handy Integrals - Colby College
www.colby.eduColby College Some Handy Integrals Gaussian Functions 0 2e–ax 2dx = 1 2 π a ½ 0 x e–ax dx = 1 2a 0 x 2 e–ax2 dx = 1 4a π a ½ 0 x 3 e–ax2 dx = 1 2a2 0 x 4 e–ax 2 dx = 3
Gibbs Phase Rule: f = c – p + 2 - Colby College
www.colby.eduColby College Gibbs Phase Rule: f = c – p + 2 f = Intensive Degrees of freedom = variance Number of intensive variables that can be changed independently without disturbing the number of phases in equilibrium p = number of phases gas, homogeneous liquid phases, homogeneous solid phases c = components Minimum number of independent constituents
Hydration of Ketones and Aldehydes - Colby College
www.colby.eduAldehydes exist as partial hydrates in aqueous solution: Ketones generally do not favor hydration: O H HO H O H H H2O H Hydration of Ketones and Aldehydes. O Me Me OH Me Me OH O Me Me Na H+ (workup) OH HONa pKa of H2O: approx. 16 pKa of alkoxide: 16-20 A hydrated ketone O Me Me Starting material! MeO Na O Me Me OH Me Me OMe O
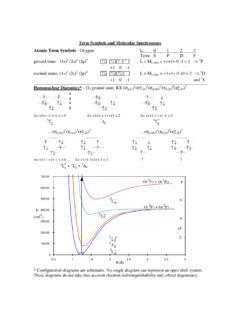
Term Symbols and Molecular Spectroscopy - Colby College
www.colby.eduTerm Symbols and Molecular Spectroscopy Atomic Term Symbols - Oxygen L 0 1 2 3 Term S P D F ground state: (1s) 2 (2s) 2 (2p) 4 ↑↓ ↑↓↑ ↑ L = M L,max = +1+1 ...
(J K - Colby College
www.colby.eduTable 7.2.1: Constant Pressure Heat Capacities for a few Substances at 298.2 K and 1 bar. 1 Substance Cp,m (J K-1 mol-1) Substance C p,m (J K-1 mol-1) He (g) 20.786 CH 4 (g) 35.309 Xe (g) 20.786 C2H6 (g, ethane) 52.63 CO (g) 29.14 C3H8 (g, propane) 73.51 CO 2 (g) 37.11 C4H10 (g, n-butane) 97.45 Table 7.2.2: Constant Pressure Heat Capacities for Different Phases. 2
Introduction to Biochemistry - Colby College
www.colby.eduExercise Physiology J. T. Millard 4 C. Like Dissolves Like Cells are pretty much tiny bags of salty water containing a lot of dissolved molecules and ions.
T V v /nR
www.colby.eduColby College Application of the First Law to Ideal Gases Calculate q,w, ∆U, and ∆H for ideal gas processes: dU = ñq + ñw dU = C v dT dH = C p dT ∆U = q + w for any process since
Capillary HPLC Introduction Capillary HPLC - Colby College
www.colby.edumodifier, followed by methanol. 2-Propanol can also be used as a modifer, however, its much higher viscosity produces higher backpressures and requires the use of shorter columns.
Exercise Physiology: Cardiovascular System - Colby College
www.colby.eduThe study of the cardiovascular exercise physiology is one of the significant disciplines of exercise physiology. It examines how oxygen and other nutrients are transported by cardiovascular system and used by the muscles during exercise.
Electrical Conductivity of Aqueous Solutions - Colby College
www.colby.eduElectrical Conductivity of Aqueous Solutions PRE-LAB Reading: Chapter sections 3.3, 3.6, 4.6, 14.5, 15.1 in Olmstead and Williams. Purpose: The predominate ions in …
Hydration of Ketones and Aldehydes - Colby College
www.colby.eduAddition of Alcohols to Carbonyl Groups: Acetal Formation Me Me O OH Me Me Me Me MeOOH a hemiacetal O Me Me O Me Me H MeO H M e Me MeOOH2 O e Me Me ROH, H+ Me Me ROOR H+ H+
Gibbs Phase Rule: f = c – p + 2
www.colby.eduColby College Gibbs Phase Rule: f = c – p + 2 f = Intensive Degrees of freedom = variance Number of intensive variables that can be changed independently without
dragging in the original spreadsheet using ... - Colby College
www.colby.eduLINEST in Excel The Excel spreadsheet function "linest" is a complete linear least squares curve fitting routine that produces uncertainty estimates for the fit values.
Chapter 4: Stereochemistry - Colby College
www.colby.eduThe CIP System Revisited MeH HMe 21 34 21 43 1. Rank each substituent attached to the stereocenter according to the CIP priority system (1 = highest, 4 = lowest)
Similar queries
Some Handy Integrals, Colby College, Colby College Some Handy Integrals, Gibbs Phase Rule, Colby College Gibbs Phase Rule, Aldehydes, Term Symbols and Molecular Spectroscopy, Term Symbols, Term, Introduction to Biochemistry, Molecules and ions, T V v /nR, Capillary HPLC Introduction Capillary HPLC, Columns, Exercise Physiology: Cardiovascular System, Cardiovascular, Physiology, Electrical Conductivity of Aqueous Solutions, Hydration of Ketones and Aldehydes, Phase Rule, Linest, Chapter 4: Stereochemistry