Search results with tag "Colby"
Some Handy Integrals - Colby College
www.colby.eduColby College Some Handy Integrals Gaussian Functions 0 2e–ax 2dx = 1 2 π a ½ 0 x e–ax dx = 1 2a 0 x 2 e–ax2 dx = 1 4a π a ½ 0 x 3 e–ax2 dx = 1 2a2 0 x 4 e–ax 2 dx = 3
Gibbs Phase Rule: f = c – p + 2 - Colby College
www.colby.eduColby College Gibbs Phase Rule: f = c – p + 2 f = Intensive Degrees of freedom = variance Number of intensive variables that can be changed independently without disturbing the number of phases in equilibrium p = number of phases gas, homogeneous liquid phases, homogeneous solid phases c = components Minimum number of independent constituents
17220 N. BOSWELL BLVD, STE 140 SUN CITY, AZ 85373-1984 …
www.colbymgt.comCOLBY MANAGEMENT 17220 N. BOSWELL BLVD, STE 140 SUN CITY, AZ 85373-1984 623-977-3860, x 7712 623-977-3577 fax E-mail form to: frontdesk@colbymgt.com
Electrical Conductivity of Aqueous Solutions - colby.edu
www.colby.eduElectrical Conductivity of Aqueous Solutions PRE-LAB ASSIGNMENT: Reading: Chapter 4.1-4.3 in Brown, LeMay, Bursten & Murphy. 1. Using Table 1 in this handout, determine which solution has a higher conductivity, 0.1 M HCl or 0.1 M
dragging in the original spreadsheet using ... - Colby College
www.colby.eduLINEST in Excel The Excel spreadsheet function "linest" is a complete linear least squares curve fitting routine that produces uncertainty estimates for the fit values.
Gibbs Phase Rule: f = c – p + 2
www.colby.eduColby College Gibbs Phase Rule: f = c – p + 2 f = Intensive Degrees of freedom = variance Number of intensive variables that can be changed independently without
1 2 3 4 5 6 - maps.tcu.edu
maps.tcu.eduN Samuelson Carter King Wright Colby Miller Sherley Starpoint/ Health Waits Landreth Foster Jarvis Scharbauer Reed Mabee Sadler Walker Britain Mullins Fish
Hydration of Ketones and Aldehydes - Colby College
www.colby.eduAddition of Alcohols to Carbonyl Groups: Acetal Formation Me Me O OH Me Me Me Me MeOOH a hemiacetal O Me Me O Me Me H MeO H M e Me MeOOH2 O e Me Me ROH, H+ Me Me ROOR H+ H+
Electrical Conductivity of Aqueous Solutions - Colby College
www.colby.eduElectrical Conductivity of Aqueous Solutions PRE-LAB Reading: Chapter sections 3.3, 3.6, 4.6, 14.5, 15.1 in Olmstead and Williams. Purpose: The predominate ions in …
Page 2BPage 2B THE NORTH GEORGIA NEWS February 9, …
nganews.comSep 22, 2002 · Davenport. Both Guild and Davenport are two of the favorites in Class AA at 160 lbs. Photo/Todd Forrest Senior Sean Phillips (L) and Fannin County’s Colby Shaw (R) clash in front of a packed house at the old Fan-nin County High School gymnasium during the 170-lb. Area title match. Photo/Todd Forrest
Hydration of Ketones and Aldehydes - Colby College
www.colby.eduAldehydes exist as partial hydrates in aqueous solution: Ketones generally do not favor hydration: O H HO H O H H H2O H Hydration of Ketones and Aldehydes. O Me Me OH Me Me OH O Me Me Na H+ (workup) OH HONa pKa of H2O: approx. 16 pKa of alkoxide: 16-20 A hydrated ketone O Me Me Starting material! MeO Na O Me Me OH Me Me OMe O
Exercise Physiology: Cardiovascular System - Colby College
www.colby.eduThe study of the cardiovascular exercise physiology is one of the significant disciplines of exercise physiology. It examines how oxygen and other nutrients are transported by cardiovascular system and used by the muscles during exercise.
Capillary HPLC Introduction Capillary HPLC - Colby College
www.colby.edumodifier, followed by methanol. 2-Propanol can also be used as a modifer, however, its much higher viscosity produces higher backpressures and requires the use of shorter columns.
T V v /nR
www.colby.eduColby College Application of the First Law to Ideal Gases Calculate q,w, ∆U, and ∆H for ideal gas processes: dU = ñq + ñw dU = C v dT dH = C p dT ∆U = q + w for any process since
Introduction to Biochemistry - Colby College
www.colby.eduExercise Physiology J. T. Millard 4 C. Like Dissolves Like Cells are pretty much tiny bags of salty water containing a lot of dissolved molecules and ions.
(J K - Colby College
www.colby.eduTable 7.2.1: Constant Pressure Heat Capacities for a few Substances at 298.2 K and 1 bar. 1 Substance Cp,m (J K-1 mol-1) Substance C p,m (J K-1 mol-1) He (g) 20.786 CH 4 (g) 35.309 Xe (g) 20.786 C2H6 (g, ethane) 52.63 CO (g) 29.14 C3H8 (g, propane) 73.51 CO 2 (g) 37.11 C4H10 (g, n-butane) 97.45 Table 7.2.2: Constant Pressure Heat Capacities for Different Phases. 2
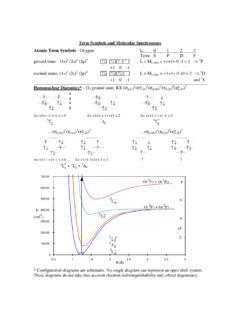
Term Symbols and Molecular Spectroscopy - Colby College
www.colby.eduTerm Symbols and Molecular Spectroscopy Atomic Term Symbols - Oxygen L 0 1 2 3 Term S P D F ground state: (1s) 2 (2s) 2 (2p) 4 ↑↓ ↑↓↑ ↑ L = M L,max = +1+1 ...
Chapter 4: Stereochemistry - Colby College
www.colby.eduThe CIP System Revisited MeH HMe 21 34 21 43 1. Rank each substituent attached to the stereocenter according to the CIP priority system (1 = highest, 4 = lowest)
Similar queries
Some Handy Integrals, Colby College, Colby College Some Handy Integrals, Gibbs Phase Rule, Colby College Gibbs Phase Rule, 17220 N. BOSWELL BLVD, STE 140 SUN, Colby, Electrical Conductivity of Aqueous, Conductivity, Linest, Phase Rule, Hydration of Ketones and Aldehydes, Electrical Conductivity of Aqueous Solutions, Davenport, Aldehydes, Exercise Physiology: Cardiovascular System, Cardiovascular, Physiology, Capillary HPLC Introduction Capillary HPLC, Columns, T V v /nR, Introduction to Biochemistry, Molecules and ions, Term Symbols and Molecular Spectroscopy, Term Symbols, Term, Chapter 4: Stereochemistry