Transcription of Yu-ran Luo
1 Bond dissociation energiesYu-ran LuoThe bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: BDE, BE, or D) . It is defined as the standard enthalpy change of the following fission: R X R + X . The BDE, denoted by Do(R X), is usually derived by the thermochemical equation, Do(R X) = fHo(R) + fHo(X) fHo(RX) . The enthalpy of formation fHo of a large number of atoms, free radicals, ions, clusters and compounds is available from the websites of NIST, NASA, CODATA, and IUPAC . Most authors prefer to use the BDE values at 298.
2 15 K . The following seven tables provide essential information of ex-perimental BDE values of R X and R+ X bonds . (1) Table 1: Bond Dissociation Energies in Diatomic Molecules(2) Table 2: Enthalpy of formation of Gaseous Atoms(3) Table 3: Bond Dissociation Energies in Polyatomic Molecules(4) Table 4: Enthalpies of formation of Free Radicals and Other Transient Species(5) Table 5: Bond Dissociation Energies of Common Organic Molecules(6) Table 6: Bond Dissociation Energies in Diatomic Cations(7) Table 7: Bond Dissociation Energies in Polyatomic CationsThe data in these tables have been revised through September 2009.
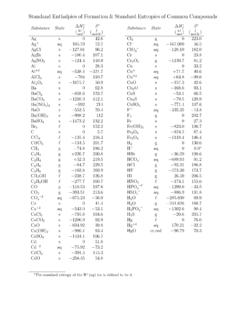
3 TaBLe 1. Bond dissociation energies in diatomic moleculesThe BDEs in diatomic species have usually been measured by spectroscopy or mass spectrometry . In the absence of data on the en-thalpy function, the values at 0 K, Do(A B), are converted to Do298 by the approximate equation: Do298(A B) Do(A B) + (3/2)RT = Do(A B) + 3 .7181 kJ mol 1 This table has been arranged in an alphabetical order of the atoms A in the diatomics A B .a BDo298/kJ mol O7941Ag Ag162 .9 2 .91Ag Al183 .7 9 .21Ag Au202 .5 9 .61Ag Bi192 421Ag Br280 .3 1 .31Ag Cl279 .1 8 .41Ag Cu171 .5 9 .61Ag D226 .81Ag Dy130 191Ag Eu127 131Ag F356.
4 9 5 .81Ag Ga159 171Ag Ge174 .5 211Ag H202 .4 9 .11Ag Ho124 191Ag I234 291Ag In166 .5 4 .91Ag Li186 .11Ag Mn99 .2 211Ag Na133 .1 12 .61Ag Nd<2131Ag O221 211Ag S216 .7 14 .61Ag Se210 .0 14 .61Ag Si185 .1 9 .61a BDo298/kJ mol Sn136 211Ag Te195 .8 14 .61Al Al264 .3 0 .51Al Ar5 .691Al As202 .7 7 .11Al Au325 .9 6 .31Al Br429 .2 5 .81Al C267 .71Al Ca52 .71Al Cl5021Al Co181 .6 0 .21Al Cr222 .9 0 .91Al Cu227 .1 1 .21Al D290 .41Al F6751Al H288 131Al I369 .9 2 .11Al Kr6 .051Al Li76 .11Al N 368 151Al Ne3 .91Al Ni224 .7 4 .81Al O501 .9 10 .61Al P216 .7 12 .61Al Pd254.
5 4 12 .11Al S332 101a BDo298/kJ mol Sb216 .3 61Al Se318 131Al Si246 .9 12 .61Al Te268 131Al Ti263 .41Al U326 291Al V147 .4 1 .01Al Xe7 .391Am O553 361Ar Ar4 .911Ar B4 .621Ar Br~5 .01Ar C5 .1581Ar Ca4 .44 0 .601Ar Cd5 .57 0 .051Ar Ga3 .961Ar Ge<5 .41Ar He3 .961Ar Hg5 .321Ar I~5 .31Ar In4 .181Ar Kr5 .111Ar Li~7 .821Ar Mg~3 .71Ar Na~4 .21Ar Ne4 .271a BDo298/kJ mol Si5 .861Ar Sn<5 .11Ar Tl4 .091Ar Xe5 .281Ar Zn5 .01As As385 .8 10 .51As Cl4481As D270 .31As F4101As Ga202 .5 4 .81As H274 .0 2 .91As I296 .6 241As In201 101As N489 2 .11As O484 81As P433 .5 12 .61As S379 .5 6.
6 31As Sb330 .5 5 .41As Se961As Tl198 .3 14 .61Au Au226 .2 0 .51Au B367 .8 10 .51Au Ba254 .8 10 .01Au Be237 .7 4 .01Au Bi293 8 .41Au Br213 211 652/23/10 5:28:50 PMa BDo298/kJ mol Ca250 .4 4 .01Au Ce322 181Au Cl280 131Au Co218 .0 16 .41Au Cr223 .7 28 .91Au Cs253 3 .51Au Cu227 .1 1 .21Au D322 .21Au Dy259 241Au Eu245 121Au F294 .11Au Fe187 .0 19 .31Au Ga290 151Au Ge273 .2 14 .61Au H300 .5 2 .64Au Ho267 351Au I2761Au In286 .0 5 .71Au La457 281Au Li284 .5 6 .71Au Lu332 191Au Mg179 .1 2 .71Au Mn197 .7 211Au Na215 .1 12 .61Au Nd294 291Au Ni247 16.
7 41Au O223 211Au Pb133 421Au Pd142 .7 211Au Pr311 251Au Rb243 3 .51Au Rh232 .6 291Au S253 .6 14 .61Au Sc280 401Au Se251 .0 14 .61Au Si304 .6 6 .01Au Sn256 .5 7 .21Au Sr264 421Au Tb285 331Au Te237 .2 14 .61Au U318 291Au V246 .0 8 .71Au Y310 121B B2901B Br390 .9 0 .51B C448 291B Cd301 .01B Ce305 211B Cl4271B D341 .0 6 .31B F7321a BDo298/kJ mol H345 .2 2 .51B I3611B Ir512 .2 171B La335 631B N377 .9 8 .71B Ne3 .971B O8091B P347 16 .71B Pd351 .5 16 .71B Pt477 .8 16 .71B Rh475 .8 211B Ru446 .9 211B S577 9 .21B Sc272 631B Se462 14 .61B Si317 121B Te354 201B Th297 331B Ti272 631B U322 331B Y289 631Ba Br4021Ba Cl4431Ba D 193.
8 71Ba F580 .61Ba H192 .01Ba I322 .6 6 .31Ba O562 13 .41Ba Pd221 .8 5 .01Ba Rh259 .4 251Ba S418 211Be Be591Be Br3161Be Cl4341Be D203 .11Be F5731Be H2211Be I2611Be O4371Be S372 591Be T204 .41Bi Bi204 .41Bi Br240 .21Bi Cl300 .4 4 .21Bi D283 .71Bi F366 .5 12 .51Bi Ga158 .6 16 .71Bi H 283 .31Bi I186 .1 5 .81Bi In153 .6 1 .71Bi Li149 .41a BDo298/kJ mol O337 .2 12 .61Bi P281 .7 131Bi Pb142 .4 3 .01Bi S315 .5 4 .61Bi Sb252 .7 3 .91Bi Se280 .3 5 .91Bi Sn193 131Bi Te232 .2 11 .31Bi Tl120 .9 12 .61Bk O5981Br Br193 .859 0 .1201Br C318 .0 8 .41Br Ca3391Br Cd159 961Br Ce373.
9 21Br Cl219 .32 0 .051Br Co326 421Br Cr328 .0 24 .31Br Cs389 .1 4 .21Br Cu331 251Br D370 .741Br Dy339 .3 10 .51Br Er361 .31Br Eu5481Br F280 121Br Fe243 841Br Ga402 131Br Gd372 .01Br Ge347 81Br H366 .16 0 .201Br Hg74 .91Br Ho321 .81Br I179 .1 0 .41Br In409 101Br K379 .1 4 .21Br La446 .21Br Li418 .8 4 .21Br Lu301 .51Br Mg317 .961Br Mn314 .2 9 .61Br Mo313 .41Br N280 .8 211Br Na363 .1 4 .21Br Nd339 .71Br Ni360 131Br O237 .6 0 .41Br P 3291Br Pb248 .5 14 .61Br Pr344 .51Br Rb380 .7 4 .21Br S218 171a BDo298/kJ mol Sb314 591Br Sc444 631Br Se297 841Br Si358 .2 8.
10 41Br Sm331 .41Br Sn337 131Br Sr3651Br T372 .771Br Tb382 .81Br Th3641Br Ti3731Br Tl331 211Br Tm299 .11Br U377 151Br V439 421Br W329 .31Br Xe5 .94 0 .021Br Y481 841Br Yb295 .41Br Zn138 291Br Zr4201C C618 .3 15 .41C Ce443 301C Cl394 .9 13 .41C D341 .41C F513 .8 10 .01C Fe376 .3 28 .91C Ge455 .7 111C H338 .4 1 .21C Hf540 251C I253 .1 35 .61C Ir631 51C La463 201C Mo482 161C N750 .0 2 .91C Nb523 .8 14 .51C Ni337 .01C O1076 .38 0 .671C Os608 251C P507 .5 8 .81C Pd436 201C Pt577 .8 6 .813C Rh580 41C Ru648 131C S713 .3 1 .21C Sc444 211C Se590 .4 5 .91C Si4471C Tc564 291C Th453 171C Ti423 3019-66 Bond dissociation 662/23/10 5:28:52 PMa BDo298/kJ mol U455 151C V423 241C Y418 141C Zr495.